
Knockdown of EphB3 inhibits cell proliferation partly through the AKT signaling pathway and represses epithelial-mesenchymal transition in esophageal squamous cell carcinoma
Introduction
Esophageal carcinoma ranks sixth for mortality and seventh for morbidity in patients worldwide (1). Esophageal squamous-cell carcinoma (ESCC) is the main histologic cell type found in about 90% of patients, especially in high-risk regions such as eastern Asia and southern Africa (2,3). Most patients present with advanced stages of the disease when diagnosed and its prognosis has improved only modestly with the development of multidisciplinary treatments, with the 5-year survival still only approximately 20% globally (3-6). It is of the utmost importance to find a novel therapy that specifically targets ESCC to improve treatment outcomes.
The erythropoietin-producing hepatocyte (Eph) receptor is the most abundant subtype of tyrosine kinase receptors. Based on their structure and ephrin ligands, Ephs are classified as EphA or EphB (7). Eph/ephrin complexes elicit bidirectional signaling and have important actions in many biological processes that include cell differentiation and motility and adhesion in addition to angiogenic actions (8,9). Ephs and ephrins dysregulation is closely related to the development of numerous diseases including cancer (8-10). However, the actions of Eph receptors in tumorigenesis are paradoxical in different cancer types. erythropoietin-producing hepatocyte receptor B3 (EphB3) is a member of EphB receptors that interact with ephrin-B ligands. Early studies have showed that EphB3 suppressed human colorectal cancer progression through enforcing E-cadherin adhesion (11,12). Our research group also found that EphB3 expression was reduced in gastric cancer and inhibited tumor progression by affecting epithelial-mesenchymal transition (13,14). In contrast, Ji et al. reported that overexpression of EphB3 occurred in non-small cell lung cancer (NSCLC) and stimulated proliferation of cells and metastasis that was kinase-independent (15). Further research has demonstrated that the phosphorylation level of EphB3 in NSCLC was low because of inadequate expression of the cognate ligands ephrin-B1 and ephrin-B2 (16). In addition, forced activation of EphB3 could suppress NSCLC cancer metastasis through actions on the PP2A/RACK1/AKT pathway (16). This perplexing dichotomy seemed to be attributed to differences in kinase activation status of the EphB3 receptor. Nemoto et al. reported that EphB3 was upregulated in esophageal squamous cell cancer (17). Nonetheless, its biological activities in ESCC remain to be unequivocally elucidated.
Therefore, we evaluated the function of EphB3 in ESCC and showed that EphB3 expression was increased in ESCC specimens and associated with patient clinicopathological features. Knockdown of EphB3 in ESCC cell lines inhibited cell growth both in vitro and in vivo. Targeting EphB3 may well represent a novel strategy for the therapy of ESCC.
We present the following article in accordance with the ARRIVE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1567/rc).
Methods
Patient tissue samples and immunohistochemistry
One-hundred pairs of paraffin-embedded ESCC and nearby normal tissue specimens were sampled from patients who underwent surgery but did not receive radio- or chemotherapy prior to resection. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2019-SRFA-002) and informed consent was taken from all individual participants. Immunohistochemistry was conducted as previously reported (13). The primary rabbit anti-EphB3 antibody (ab135809) (Abcam, Cambridge, UK) was 1:100 diluted. The IHC slides were assessed by two pathologists who were blind to clinical information. For statistical analysis, the intensity and percentage of cells stained was determined and scored as previously described (18). The staining intensity score ranges were: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The points representing the percentage of stained cells were: 0, 0–5%; 1, 6–25%; 2, 26–50%; 3, 51–100%. The total histological scores ranging from 0 to 6 was the result of the addition sum of 2 primary scores. A histological score of 0–2 was considered to be negative or low positive, a histological score of 3–4 was regarded as being moderately positive and a histological score >4 was considered to be highly positive expression of EphB3.
Cell culture and EphB3 gene knockdown
HEEC cells (BNCC337729) were supplied by the Beina Chuanglian Biotechnology Institute (Beijing, China). The ESCC cell line TE-1 (TCHu89) was sourced from the Cell Bank of the Chinese Academy of Sciences and TE10, TE-13, KYSE-150, KYSE-450 were gifts from Professor Sun of Nanjing Medical University. Dulbecco’s modified Eagle’s medium (containing 10% fetal bovine serum and 1% penicillin and streptomycin) was used to culture the cells in reagents and supplements supplied by GIBCO Invitrogen Inc. (Carlsbad, CA, USA). The ESCC cells were treated with 5 µg/mL AKT activator SC79 (ab 146428, Abcam) for 48 h. To generate cell lines that stably suppressing EphB3, EphB3-targeting shRNA or scrambled shRNA sequences were cloned into the linear lentiviral vector pgLV3/H1/GFP + Puro Vector provided by GenePharma (Shanghai); cells were then infected with the recombinant lentivirus. Next, they were selected after the addition of puromycin (2 µg/mL) to the culture medium for 14 days. shRNAs sequences were designed using software provided by Invitrogen and details are listed in Table S1.
qRT-PCR analysis
Total cell RNA content was extracted using Trizol (Invitrogen). 1 µg aliquots of the RNA were reverse transcribed to cDNA with a PrimeScript™ RT reagent Kit (TaKaRa, China). qRT-PCR in a final volume of 20 µL was conducted using TB Green™ Premix Ex Taq™ and a ABI 7300 PCR system (Applied Biosystems, USA). Data were evaluated using the 2−ΔΔCq technique. Primers were synthesized by TaKaRa and the 5' to 3' sequences were as follows: EphB3-F: TGGGTAACATCTGAGTTGGCG, EphB3-R: TGGTATGTGCGGATGGGATTC; GAPDH-F: GCTCTCTGCTCCTCCTGTTC; GAPDH-R ACGACCAAATCCGTTGACTC.
Western blotting
Proteins were isolated from cultured cells using the Whole Cell Lysis Assay kit (KeyGen BioTech, Nanjing), which contained proteinase and various phosphatase inhibitors. Protein concentrations for each sample were evaluated with a BCA protein assay kit (Beyotime, Beijing), separated using SDS-PAGE. They were next placed on a PVDF membrane (Millipore, USA), and exposed to appropriate specific antibodies. An electrochemiluminescence chromogenic substrate (Thermo Fisher Scientific, USA) was used to see the various protein bands. The primary antibodies used were: anti-EphB3 (ab135809), anti-AKT (ab8805) from Abcam; anti-E-cadherin (3195T), anti-N-cadherin (13116T); anti-Slug (9585T); anti-bax (2774s); and anti-caspase-3 (9662s), all provided by CST (Cell Signaling Technology, USA); anti-vimentin (sc6260) and anti-bcl2 (sc-509) from Santa Cruz (Santa Cruz Biotechnology, Inc., Santa Cruz, USA); anti-pAKT-S473 (66444-1-lg), anti-cyclinD1 (60186-1-lg) from Proteintech (Proteintech, Manchester, UK). The secondary antibodies employed were goat anti-mouse (SA00001-1) and goat anti-rabbit (SA00001-2) (Proteintech).
CCK-8 assay
A counting kit-8 (CCK-8) (Bimake, USA) was employed to determine the proliferation of cells after 4×103 cells per well were seeded into each plate. Absorbance was measured at 570 nm using an ELx800 microplate reader (Bio-Tek, USA).
Transwell assay
For cell migration assays, 300 µL of serum-free medium containing 6×104 cells was seeded into the upper chamber of uncoated Transwell. In the Matrigel upper chamber, to determine the degree of cell invasion, 300 µL of a serum-free medium containing 1×105 cells was seeded into each well (BD Biosciences, Franklin Lake, NJ, USA) on coated Transwell. A 500 µL volume of DMEM with 20% FBS added was added to the lower chamber. After 24 h of culture, cells were stained with crystal violet (0.1%), and cells in the upper membrane carefully collected. The lower layer of migrating/invading cells were observed on an IX71 inverted Olympus microscope and digital images were recorded.
Wound healing assay
For cells seeded into 6-well plates, after they had spread over 80–90% of the plate bottoms, scratch wounds were created using a small 10 µL pipette tip. The region filled by cells that had migrated was digitally recorded at intervals from 0 to 36 h and measured using the Digimizer software system.
Colony formation assay
For this assay, cells were seeded (200 cells/well) in triplicate. After 14 days the colonies formed were fixed in 10% formaldehyde for 5 min and subsequently stained for 30 s with crystal violet (1%). Colonies containing >50 cells were analysed.
Flow cytometry analysis
Approximately 8×104 cells were seeded in triplicate into plates and harvested after 72 h of culture. To determine the degree of apoptosis, cells were incubated for 15 min with propidium iodide and Annexin V-FITC (Beyotime, Shanghai, China) followed by analysis using flow cytometry (Beckman, USA). For the analysis of the cell cycle, cells were fixed in 70% ethanol overnight at 4 °C. They were then incubated with propidium iodide for 30 min at 37 °C in the dark. Finally, the cells were analysed using flow cytometry within 2 h of preparation.
Xenograft tumor experiments
BALB/c nude mice (females, 4–5 weeks old, weight range 18 to 20 g) were sourced from the Nanjing Medical University Animal Center and housed here in a specific pathogen-free environment. The animal studies were performed under a project license (No. IACUC 1706007) granted by the ethics committee of Nanjing Medical University, in compliance with the national and institutional guidelines for the care and use of animals. These mice were randomly divided into two groups (4 per group). Then xenograft tumor models were established by inoculating 0.1 mL (1×107 cells/mL) of Ephb3 knockdown TE-1 cells and TE-1 cells, which served as the control, subcutaneously into the left forelimb armpit. The sizes of tumor were measured at 3-day intervals with calipers, and the tumor volume (V) was calculated: [(smaller diameter)2 × (longer diameter) / 2]. Tumors were excised and weighed 3 weeks after cell inoculation and fixed in ice-cold neutral buffered formalin (10%) for immunohistochemistry. The histological scores of EphB3 were calculated by: (cell number of negative stain × 0 + cell number of weak staining × 1 + cell number of moderate staining × 2 + cell number of strong staining × 3) / total cell number.
Statistical analysis
Research data analyzes were conducted using SPSS ver. 22.0. Differences in the expression of EphB3 in tissue samples and the correlation between EphB3 expression and various clinicopathological parameters were evaluated using the chi-squared test. Overall survival analysis was evaluated by Kaplan-Meier method with the log-rank test. Univariate and multivariate Cox regression analyses were used to assess survival data. All measured data from in vitro and in vivo experiments are presented as means ± SD and compared for significant differences using an unpaired Student’s t-test. A P value <0.05 was deemed to be a noteworthy result.
Results
EphB3 was significantly expressed at high levels in ESCC and predicted a poor prognosis
The expression status of EphB3 protein in 100 pairs of ESCC and nearby normal tissue was analyzed using immunohistochemistry methods. The protein was generally detected as brown particles in the cytoplasm. EphB3 expression was highly positive in 74 (74%) ESCC specimens, while in normal tissue, EphB3 was only highly expressed in 23 (23%) cases (P<0.01) (Table 1). EphB3 was significantly increased in ESCC compared to normal tissue (Figure 1). The relation between expression of EphB3 and clinicopathological findings in the patients was also investigated (Table 2). A positive relation was found between the EphB3 expression level and metastasis of lymph nodes (P<0.05), TNM stage (P<0.05). In addition, strong positive EphB3 expression was more common in poorly differentiated tumors (P<0.05). No association was found between EphB3 expression level and gender, age, depth of tumor invasion, and tumor location.
Table 1
| Groups | Cases | EphB3 expression | χ2 | P value | ||
|---|---|---|---|---|---|---|
| Negative + low | Moderate | High | ||||
| ESCC | 100 | 1 | 25 | 74 | 55.119 | 0.000 |
| Adjacent normal tissues | 100 | 16 | 61 | 23 | ||
ESCC, esophageal squamous-cell carcinoma.
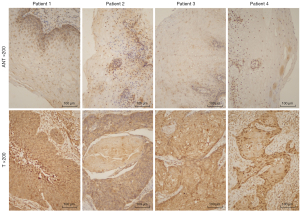
Table 2
| Characteristic | Cases (n=100) | EphB3 expression | χ2 | P value | |
|---|---|---|---|---|---|
| Negative + low + moderate | High | ||||
| Gender | 0.585 | 0.488 | |||
| Male | 63 | 18 | 45 | ||
| Female | 37 | 8 | 29 | ||
| Age | 0.923 | 0.364 | |||
| <65 years | 58 | 13 | 45 | ||
| ≥65 years | 42 | 13 | 29 | ||
| Depth of invasion | 0.330 | 0.651 | |||
| T1–T2 | 49 | 14 | 35 | ||
| T3–T4 | 51 | 12 | 39 | ||
| Lymph node† | 7.433 | 0.011 | |||
| Negative | 54 | 20 | 34 | ||
| Positive | 46 | 6 | 40 | ||
| TNM stage | 6.883 | 0.011 | |||
| I–II | 59 | 21 | 38 | ||
| III–IV | 41 | 5 | 36 | ||
| Differentiation | 7.072 | 0.029 | |||
| Grade 1 | 24 | 11 | 13 | ||
| Grade 2 | 38 | 9 | 29 | ||
| Grade 3 | 38 | 6 | 32 | ||
| Location | 2.073 | 0.462 | |||
| Upper | 6 | 3 | 3 | ||
| Middle | 52 | 13 | 39 | ||
| Low | 42 | 10 | 32 | ||
†, lymph node status: negative, no positive nodal metastases; positive, number of positive nodal metastases ≥1. ESCC, esophageal squamous-cell carcinoma.
To further explore the correlation between EphB3 expression and patient prognosis, Kaplan-Meier survival analysis was conducted, and the results indicated that patients with high EphB3 expression had worse overall survival (OS) (P=0.004). The 3-year OS rate was 16.2% in the high EphB3 expression group, whereas 42.3% in the low EphB3 expression group (Figure 2A). Univariate and multivariate Cox regression analysis revealed that EphB3 expression could act as a prognosis predictor in ESCC (Table 3).

Table 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Gender | 1.128 (0.711–1.789) | 0.610 | |||
| Age | 0.999 (0.635–1.573) | 0.997 | |||
| Depth of invasion | 1.192 (0.761–1.866) | 0.443 | |||
| Lymph node | 2.723 (1.706–4.346) | 0.000 | 2.425 (1.502–3.916) | 0.000 | |
| TNM stage | 2.423 (1.528–3.843) | 0.000 | |||
| Differentiation | 1.241 (0.786–1.960) | 0.353 | |||
| Location | 0.957 (0.609–1.504) | 0.849 | |||
| Expression of EphB3 | 2.225 (1.259–3.933) | 0.006 | 1.800 (1.002–3.232) | 0.049 | |
ESCC, esophageal squamous-cell carcinoma; CI, confidence interval; HR, hazard ratio.
Western blots and qRT-PCR were conducted to measure EphB3 expression in HEEC and ESCC cell lines including TE-1, TE-10, TE-13, KYSE-150 and KYSE-450. The experiments revealed that EphB3 was expressed to a much greater degree in ESCC in comparison to HEEC cells (Figure 2B,2C).
EphB3 knockdown inhibited the proliferation, migration and invasion of ESCC cells
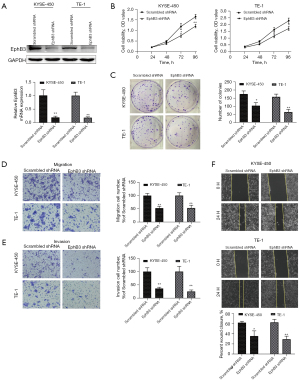
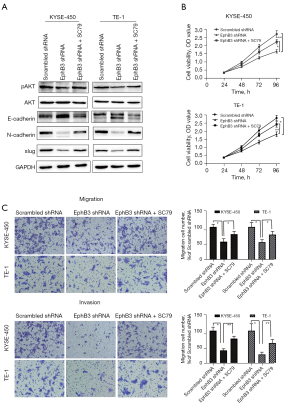
To investigate the actions of EphB3, the gene was knocked down in KYSE-450 and TE-1 cells using targeting shRNA (Figure 3A). The CCK-8 assay revealed that proliferation of cells was markedly reduced in EphB3 knockdown KYSE-450 and TE-1 cells compared to cells transfected with scrambled shRNA (Figure 3B). We also discovered that EphB3 knockdown inhibited colony formation in both KYSE-450 and TE-1 cells (Figure 3C). The wound healing assays and Transwell assays indicated that lower EphB3 expression was correlated with slower migration and invasion rates compared to scrambled shRNA cells (Figure 3D-3F). Thus, knockdown of EphB3 inhibited cell growth and restrained cell invasion and migration in ESCC in vitro.
EphB3 knockdown repressed cell cycle progression
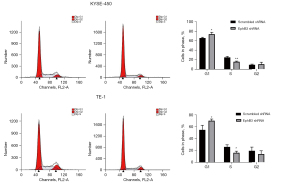
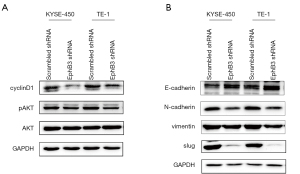
Flow cytometry detection was conducted to evaluate the effects of EphB3 on progression of the cell cycle and the degree of apoptosis of ESCC cells. As shown in Figure 4, EphB3 knockdown dramatically increased the percentage of KYSE-450 and TE-1 cells in G1 phase, consistent with the finding that decreased EphB3 expression suppressed ESCC cell proliferation. In addition, expression of the cell cycle regulatory proteins phosphorylated AKT and cyclinD1 was decreased in KYSE-450 and TE-1 cells after EphB3 knockdown (Figure 5A). However, flow cytometry results indicated that EphB3 knockdown had no significant effect on apoptosis of KYSE-450 and TE-1 cells or expression of apoptosis-related proteins (e.g., Bcl-xl, Bax or Bcl-2) (Figure S1).
EphB3 knockdown attenuated EMT via AKT pathway
To explore whether EphB3 knockdown impacted EMT activity, we detected the EMT markers using western blotting. The results clearly showed that suppressed EphB3 expression promoted the E-cadherin expression and decreased N-cadherin, Vimentin, and Slug protein expression in ESCC cells (Figure 5B). Furthermore, the effects of EphB3 knockdown on EMT and cell proliferation, migration and invasion were blocked by activation of AKT by SC79 (Figure 6A-6C). Therefore, it was supposed that EphB3 knockdown could suppress the proliferation and metastasis by blocking AKT pathway.
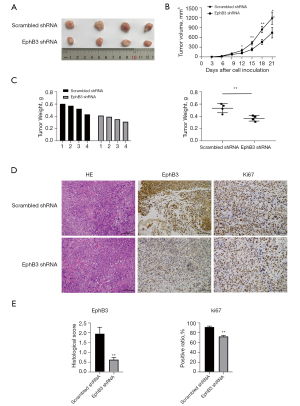
EphB3 knockdown suppressed tumor growth in vivo
To evaluate whether inhibition of EphB3 had a similar effect on the growth of tumors as shown in vitro, a mouse xenograft model was constructed and tumor growth was monitored every 3 days for 3 weeks. As shown in Figure 7, the sizes and weights of tumors formed by EphB3-silenced TE-1 cells were smaller than those formed by scrambled shRNA transfected TE1 cells (P<0.05). Immunohistochemical analysis revealed that the EphB3 gene was effectively knocked down and exhibited lower Ki67 expression. These results unequivocally demonstrated that inhibition of EphB3 could suppress tumor growth in vivo.
Discussion
Much recent research has revealed that EphB3 is dysregulated in a wide variety of human cancers, participates in carcinogenesis and progression, and that its functions are complex depending on the type of cancer (10-16). Over expression of EphB3 in cancer of esophageal squamous cells has been reported (17) and consistent with this latter finding, we demonstrated that EphB3 was more highly expressed in ESCC specimens compared to nearby normal tissue. In addition, a high EphB3 expression level was positively correlated with poor prognosis and clinicopathological features such as tumor differentiation, metastasis into regional lymph node and the TNM stage. Next, additional experiments revealed that EphB3 expression was also increased in ESCC cells, but low in HEEC cells. Knockdown of EphB3 in ESCC cells not only retarded in vitro proliferation, migration and invasion of cells, but also inhibited the growth of tumors in vivo. Similar to our findings, Li et al. reported that EphB3 was upregulated in papillary thyroid cancer and could stimulate cell migration and tumorigenesis (19). Our study suggested that EphB3 may exert a tumor-promoting effect during ESCC cell progression.
We also found that depressed expression of EphB3 blocked cell cycle progression and induced G1/G0 phase arrest, which could explain the cell growth suppression effect of EphB3 inhibition in ESCC cells. A similar function of EphB3 has been previously reported, which showed that silencing this gene decreased the numbers of NSCLC cells in S-phase, as well as the expression of PCNA and cyclinD1 (15). Another research has indicated that METTL3 could promote cell proliferation via activation of the AKT pathway and decreased expression of its downstream effectors p70 and cyclinD1 in ESCC (20). As shown in previous studies, EphB3 could affect cell proliferation through AKT pathway (21,22), therefore, we further explored the phosphorylation level of AKT to investigate the molecular mechanism involved. We discovered that silencing EphB3 produced a pronounced decrease in the expression of p-AKT and the downstream protein cyclin D1 in KYSE-450 and TE-1 cells. Our results indicated that the anti-proliferative effect of EphB3 downregulation in ESCC was associated with arrest of the cell cycle through AKT signaling.
Epithelial-mesenchymal transition (EMT) is known to be intimately associated with the migration, invasion and metastasis, which are the main factors causing high mortality in EC patients (23-25). In our study we detected an increased level of E-cadherin and decreased levels of N-cadherin, Vimentin and Slug in ESCC cells after EphB3 knockdown. Lee et al. also found that decreased EphB3 activity could reduce EMT in FGFR inhibitor resistant gastric cancer cells (26). Since EMT was looked on as a critical downstream effect of the AKT signaling axis (27), we elevated AKT phosphorylation level by its activator SC79 and found the suppressed effects of EphB3 knockdown on EMT was reversed, as well as the inhibitory effect on cell migration and invasion. Therefore, we confirmed that EphB3 inhibition could restrict tumor migration and invasion in ESCC carcinoma through regulating EMT via AKT pathway.
Conclusions
Our research demonstrated that EphB3 was markedly expressed in ESCC cells and tissue. EphB3 silencing was anti-correlated with the growth, migration and invasiveness of cells. The data suggested that EphB3 plays a vital role in the process of tumorigenesis and may be a potential novel target for ESCC therapy.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81672896) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1567/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1567/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1567/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1567/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2019-SRFA-002) and informed consent was taken from all individual participants. The animal studies were performed under a project license (No. IACUC 1706007) granted by the ethics committee of Nanjing Medical University, in compliance with the national and institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360-73. [Crossref] [PubMed]
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383-96. [Crossref] [PubMed]
- Wang VE, Grandis JR, Ko AH. New Strategies in Esophageal Carcinoma: Translational Insights from Signaling Pathways and Immune Checkpoints. Clin Cancer Res 2016;22:4283-90. [Crossref] [PubMed]
- Fatehi Hassanabad A, Chehade R, Breadner D, et al. Esophageal carcinoma: Towards targeted therapies. Cell Oncol (Dordr) 2020;43:195-209. [Crossref] [PubMed]
- Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 2020;50:12-20. [Crossref] [PubMed]
- Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell 1997;90:403-4. [Crossref] [PubMed]
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer 2010;10:165-80. [Crossref] [PubMed]
- Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol 2016;17:240-56. [Crossref] [PubMed]
- Anderton M, van der Meulen E, Blumenthal MJ, et al. The Role of the Eph Receptor Family in Tumorigenesis. Cancers (Basel) 2021;13:206. [Crossref] [PubMed]
- Batlle E, Bacani J, Begthel H, et al. EphB receptor activity suppresses colorectal cancer progression. Nature 2005;435:1126-30. [Crossref] [PubMed]
- Cortina C, Palomo-Ponce S, Iglesias M, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet 2007;39:1376-83. [Crossref] [PubMed]
- Xu TP, Wang WY, Ma P, et al. Upregulation of the long noncoding RNA FOXD2-AS1 promotes carcinogenesis by epigenetically silencing EphB3 through EZH2 and LSD1, and predicts poor prognosis in gastric cancer. Oncogene 2018;37:5020-36. [Crossref] [PubMed]
- Zhao K, He J, Wang YF, et al. EZH2-mediated epigenetic suppression of EphB3 inhibits gastric cancer proliferation and metastasis by affecting E-cadherin and vimentin expression. Gene 2019;686:118-24. [Crossref] [PubMed]
- Ji XD, Li G, Feng YX, et al. EphB3 is overexpressed in non-small-cell lung cancer and promotes tumor metastasis by enhancing cell survival and migration. Cancer Res 2011;71:1156-66. [Crossref] [PubMed]
- Li G, Ji XD, Gao H, et al. EphB3 suppresses non-small-cell lung cancer metastasis via a PP2A/RACK1/Akt signalling complex. Nat Commun 2012;3:667. [Crossref] [PubMed]
- Nemoto T, Ohashi K, Akashi T, et al. Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology 1997;65:195-203. [Crossref] [PubMed]
- Xuan Z, Huang J, Gao L, et al. Receptor Tyrosine Kinase EphB3: a Prognostic Indicator in Colorectal Carcinoma. Pathol Oncol Res 2020;26:541-9. [Crossref] [PubMed]
- Li JJ, Sun ZJ, Yuan YM, et al. EphB3 Stimulates Cell Migration and Metastasis in a Kinase-dependent Manner through Vav2-Rho GTPase Axis in Papillary Thyroid Cancer. J Biol Chem 2017;292:1112-21. [Crossref] [PubMed]
- Hou H, Zhao H, Yu X, et al. METTL3 promotes the proliferation and invasion of esophageal cancer cells partly through AKT signaling pathway. Pathol Res Pract 2020;216:153087. [Crossref] [PubMed]
- Maddigan A, Truitt L, Arsenault R, et al. EphB receptors trigger Akt activation and suppress Fas receptor-induced apoptosis in malignant T lymphocytes. J Immunol 2011;187:5983-94. [Crossref] [PubMed]
- Wen Z, Liu Q, Wu J, et al. Fibroblast activation protein α-positive pancreatic stellate cells promote the migration and invasion of pancreatic cancer by CXCL1-mediated Akt phosphorylation. Ann Transl Med 2019;7:532. [Crossref] [PubMed]
- Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016. Cell 2016;166:21-45. [Crossref] [PubMed]
- Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol 2019;29:212-26. [Crossref] [PubMed]
- Niwa Y, Yamada S, Koike M, et al. Epithelial to mesenchymal transition correlates with tumor budding and predicts prognosis in esophageal squamous cell carcinoma. J Surg Oncol 2014;110:764-9. [Crossref] [PubMed]
- Lee SY, Na YJ, Jeong YA, et al. Upregulation of EphB3 in gastric cancer with acquired resistance to a FGFR inhibitor. Int J Biochem Cell Biol 2018;102:128-37. [Crossref] [PubMed]
- Karimi Roshan M, Soltani A, Soleimani A, et al. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie 2019;165:229-34. [Crossref] [PubMed]


