
Open and minimally invasive pancreatic surgery—a review of the literature
Background
Minimally invasive gastrointestinal surgery has demonstrated reduced post-operative pain, shorter hospital stays, rapid return to baseline performance status, and reduced morbidity with oncological equivalent outcomes when compared to the traditional open procedures (1-11). However, adoption of laparoscopy for the pancreas has been slower to evolve due to the retroperitoneal position, proximity of major vascular structures, delicate nature of the organ, technical challenges of reconstruction and tendency for post-operative complications that can result in significant morbidity.
In 1994, Gagner and Pomp described the first laparoscopic pancreaticoduodenectomy in a patient with chronic pancreatitis and concluded that while it was technically feasible, the laparoscopic procedure may not improve the post-operative outcome or shorten the post-operative recovery period (12). In 1996, Gagner and Pomp also reported on their initial experience with laparoscopic distal pancreatectomy (LDP) in patients with islet cell tumors and concluded that laparoscopic resection resulted in shorter hospital recovery and is a feasible alternative to open surgery (13).
Since these first reports there is increasing evidence demonstrating not only the safety and feasibility of laparoscopic pancreatic resection, but that it may also result in enhanced postoperative recovery. Current techniques employed for minimally invasive pancreatic resection include total laparoscopy and robotic-assisted laparoscopy. The purpose of this study is to review and analyze recent advances in LDP and minimally invasive pancreaticoduodenectomy (MIPD) with an emphasis on laparoscopic technique, intraoperative outcomes, perioperative outcomes, and oncologic outcomes.
Methods
Relevant publications were identified by searching the following databases: MEDLINE (via PubMed, Ovid MEDLINE, Ovid MEDLINE In-Process & Other Non-Indexed Citations, and Ovid MEDLINE Daily), Embase (via Embase.com), and Web of Science. Publication date was limited from January 2005 to articles indexed in the databases as of August 2015. The final search was completed on August 25, 2015. No language limits were applied. Animal studies, comments, editorials, and letters were excluded. The search strategies included the following concepts: “pancreatic neoplasms”, “total pancreatectomy”, “distal pancreatectomy”, and “pancreaticoduodenectomy.” Multiple subject headings (including MeSH [Medical Subject Headings] terms in MEDLINE and Emtree terms in Embase) and text words were used to identify each concept and develop the search strategies. The following is an example of the search strategy used in PubMed: Pancreatic cancer concept = (pancrea* AND (cancer* OR tumor* OR tumour* OR neoplas* OR carcinoma* OR adenocarcinoma* OR cholangiocarcinoma* OR malignan* OR oncolog*)) OR “Pancreatic Neoplasms”[Mesh] OR (“Pancreas”[Mesh] AND “Neoplasms”[Mesh]); pancreaticoduodenectomy concept = (whipple* OR pancreaticoduodenectom* OR pancreatoduodenectom* OR “Pancreaticoduodenectomy”[Mesh]) AND open AND (laparoscop* OR “minimally invasive”); distal pancreatectomy concept = (pancreatectom* OR splenopancreatectom* OR “Pancreatectomy”[Mesh]) AND (distal OR left) AND open AND (laparoscop* OR “minimally invasive”); total pancreatectomy concept = (pancrea* AND resect*) OR pancreatectom* OR splenopancreatectom* OR “Pancreatectomy”[Mesh]) AND total AND open AND (laparoscop* OR “minimally invasive”).
Relevant articles identified by cross-referencing were also reviewed. Studies were included only if they were original series in adult patients comparing laparoscopic and open distal pancreatectomy or pancreaticoduodenectomy in the English language. Hand-assisted techniques were excluded. The laparoscopic group included at least 10 patients to minimize the effect of the learning curve for the technique. Patients were also excluded if the study was not original data, a review article, non-English, at least one of the outcomes of interest were not included, or animal studies.
Variations in the laparoscopic technique in the pancreaticoduodenectomy group included total MIPD and robotic pancreaticoduodenectomy (RPD). Total laparoscopic was defined by completely laparoscopic resection of the head of the pancreas and duodenum, followed by completely intra-corporeal reconstruction of the biliary, pancreatic, and intestinal continuity.
The outcomes of interest were: patient demographics (age, male gender, BMI, malignancy, and tumor size), intraoperative variables (operative time, blood loss, blood transfusions, conversion rate), oncologic variables [number of lymph nodes (LNs), number of patients with positive lymph nodes, and margin positivity], postoperative morbidity and mortality (overall morbidity, 30 day or in-hospital mortality, pancreatic fistula, delayed gastric emptying (DGE), bile leak, bleeding, and wound infections), and post-operative outcomes (length of hospital stay, readmission rates, reoperation rates, time to return of bowel function, time to oral intake, time to ambulation, analgesic requirements, and total hospital cost).
Odds ratios (ORs) were calculated from dichotomous data and the mean difference (MD) from the continuous data, both with 95% confidence intervals (CI). An OR less than 1 represented a more favorable outcome with laparoscopic surgery. Reported medians and ranges were used to estimate means and standard deviations using the method proposed by Hozo et al. (14). Heterogeneity was determined among the trials using the Cochrane Q-test (n-1 degree of freedom; P<0.05 to denote statistical significance). I2 was calculated to measure the proportion of total variation in the estimates of treatment effect attributable to heterogeneity beyond chance. If heterogeneity was detected (Q-test, P<0.10, or I2>50%), a random-effects model was applied. Otherwise, a fixed-effects model was used. Meta-regression was used to estimate the extent to which measured covariates (year of study, sample size, ASA of ≥3, malignancy, and tumor size) could explain the observed heterogeneity in the outcomes. Statistical analysis was performed using OpenMeta[Analyst](15).
Results
Laparoscopic distal pancreatectomy (LDP)
Selected studies
A total of 495 articles were reviewed and this analysis pooled data from 42 studies published between 2006 and 2015, which included 18,587 patients. In total, 3,759 were allocated to the LDP group and 14,828 to the open distal pancreatectomy group. No prospective randomized controlled trials were identified.
Patient selection
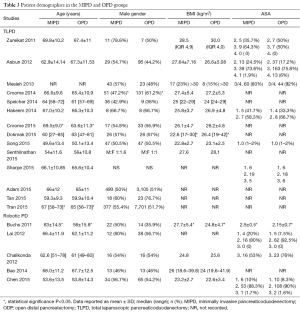
The mean age of the patients in the LDP group was 54.9±10.8 and 58.4±5.3 years in the open pancreaticoduodenectomy (OPD) group. In the LDP group, 40.0% (n=1,353) of patients were males and 44.1% (n=6,035) in the ODP group. In the LDP group, 40.4% (n=310) of patients had an ASA of ≥3 and 68.2% (n=524) in the OPD group. The indication for operation was malignancy in 27.8% (n=914), benign/premalignant cystic disease in 14.1% (n=464), benign conditions in 43.5% (n=1,432), and neuroendocrine tumors (NETs) in 14.7% (n=483) in the LDP group and malignancy in 34.8% (n=5,031), benign/premalignant cystic disease in 4.5% (n=647), benign conditions in 55.0% (n=794), and NET in 5.7% (n=821) in the ODP group. The mean tumor size in the LDP group was 3.59±0.83 and 4.6±1.3 cm. The contraindications for minimally invasive distal pancreatectomy were rarely reported, but included patient (16-19) and surgeon preference (16,20-23), malignancy (24), and contraindications to laparoscopy (19) (see Tables 1,2).
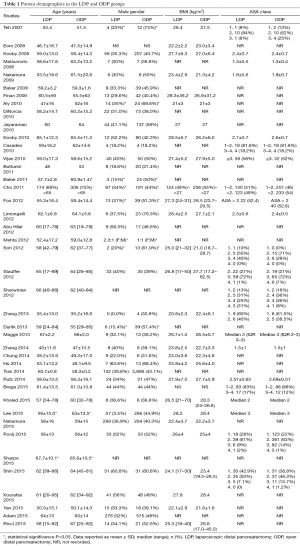
Full table
Full table
Intra-operative considerations
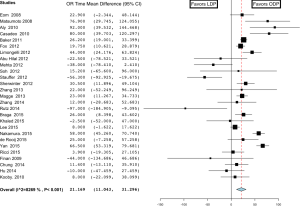
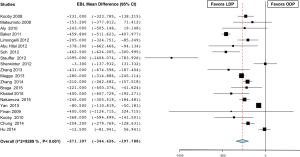
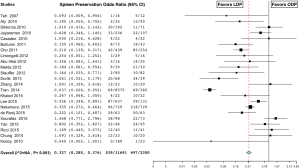
The mean operative time was 231±62.8 minutes in the LDP group versus 216.5±55.0 minutes in the ODP group. Operating room time was longer in the LDP group in 8 studies (25-32), shorter in 4 studies (33-36), and similar in 18 studies (16,18,19,21-23,37-48). Overall, the analysis showed that operating room time was statistically significantly longer in the LDP group (MD 21.169; 95% CI, 11.043 to 21.296) (Figure 1). There was a high level of heterogeneity (I2=82.7%) across studies and subsequent meta-regression analysis indicated that ASA of ≥3 (P=0.034) might be a significant explanation for some of the heterogeneity. The mean estimated blood loss was 276±102.8 cc in the LDP group and 580±280.7 cc in the ODP group. Estimated blood loss (MD −274.553; 95% CI, −351.646 to -197.460) (Figure 2) (16,18,21,23,25,26,29-32,34,37,38,42,44,46-48) and the number of red blood cell transfusions (OR 0.562; 95% CI, 0.416 to 0.760, fixed-effects) (Figure 3) (16,18,22,26,29-31,35,38,40-42,44,47,49) were statistically significantly lower in the LDP group. The spleen was preserved in 29.9% of patients in the LDP group and 14.4% in the ODP group, which reached statistical significance (OR 0.327; 95% CI, 0.285 to 0.376) (Figure 4) (16,17,19-22,24,25,27-29,31-33,36,38,39,42,43,45,48,50,51). There was a high level of heterogeneity among studies evaluating blood loss (I2=93.3%) and splenic preservation (I2=90.3%). Meta-regression analysis indicated that malignancy (P<0.001) and tumor size (P=0.029) might be a significant explanation for some of the heterogeneity in the outcome splenic preservation; however, no covariates were able to explain the heterogeneity in blood loss. Conversion rate was reported in 26 articles (n=1,814, 60.5%). LDP was converted to an open procedure in 17.6% of cases (n=320), most commonly due to bleeding (n=51, 15.9%), adhesions (n=19, 5.9%), vessel involvement (n=18, 5.6%), and lack of progress (n=15, 4.7%) (17,18,21,25-27,29,30,33-36,38,39,41-44,48,51-55).

Oncologic outcomes
The mean number of LNs retrieved was 11±4.5 in the LDP group versus 12.8±3.2 in the ODP group, which did not reach statistical significance (MD −1.636; 95% CI, −4.893 to 1.622) (18,21,23,26,35,37,39,43,45,47,48,52,56). The number of patients with positive LNs in the LDP group was 29.6% versus 36.8% in the ODP group and was not significant (OR 0.951; 95% CI, 0.710 to 1.273, fixed-effect model) (18,23,33,44,51,56) in the LDP versus ODP group. There was a high level of heterogeneity among studies evaluating the total number of LNs (I2=97.4%) and no covariate on meta-regression analysis explained the heterogeneity significantly; however, there was a trend with malignancy (P=0.066). The rate of positive margins was 6.1% in the LDP group and 12.3% in the ODP group. The LDP group had a statistically significant lower positive margin rate than the ODP group (OR 0.569; 95% CI, 0.422 to 0.768, fixed effect model) (Figure 5) (16,21,23,26,28,29,33,43,44,51,56).

Morbidity and mortality
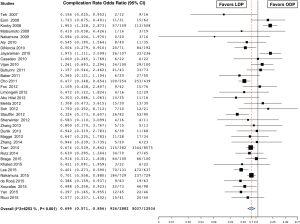
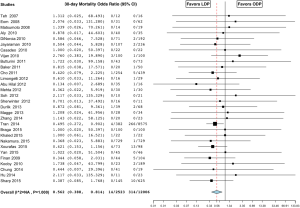
The rate of overall complications was 32.1% in the LDP group and 40.2% in the ODP group. Complication rates were lower in the LDP group in 9 studies (20,31-33,36,43,50,51,53) and no different in the remaining 25 studies (16,17,19,22-31,35,37,39-42,44-49,55). Overall, the analysis showed a significantly lower complication rate in the LDP group (OR 0.699; 95% CI, 0.571 to 0.856, P<0.001, I2=62.5%) (Figure 6). In-hospital or 30-day mortality was 0.6% in the LDP group and 2.6% in the ODP group. Fourteen studies reported zero percent mortality in both groups (18,23-27,30,32,36,38,40,44,46,47). Although not statistically significant, mortality was higher in the LDP group in one study (55) and lower in 14 studies (16,17,20,21,28,29,31,33,34,37,45,50,51,57). Overall, the analysis showed a significantly lower 30-day mortality or in-hospital mortality in the LDP group (OR 0.562; 95% CI, 0.388 to 0.814, P=0.002, fixed effect model) (Figure 7). There was a high level of heterogeneity among studies evaluating overall complication rates (I2=62.5%) and meta-regression analysis indicated that year of surgery (P=0.001) might be a significant explanation for some of the heterogeneity. Thirty-three percent of patients in the LDP group developed a pancreatic fistula versus 26% in the ODP group and only one study reported a significantly higher pancreatic fistula rate in the LDP group (28.5% versus 13.3%) (41). Although not statistically significant, the rate of pancreatic fistula was lower in the LDP group in 19 studies (16,18,20,22,23,25-27,29,32,33,37,39,43,44,48,49,51,53), higher in 13 studies (17,19,24,28,31,34,36,38,40,42,45,47,55,58), and equivalent in 2 studies (35,54). Overall, the analysis showed a statistically similar rate of pancreatic fistula in the two groups (OR 1.040; 95% CI, 0.917 to 1.181, P=0.539, fixed effect model). There was no difference in the need for re-operation between the groups (OR 0.823; 95% CI, 0.546 to 1.242, P=0.354, fixed effect model) (18-20,22,24,26,28-30,32,33,36,41,42,44,45,48,51,53). In the LDP group, 5.1% of patients had bleeding post-operatively versus 18.2% in the ODP group; however, this did not reach significance (OR 1.269; 95% CI, 0.546 to 2.948, P=0.579, fixed effect model) (17,19,24,26,29,31,32,39,47,48,50). Patients in the LDP group had a wound infection rate of 1.9% versus 2.3% in the ODP group, which was significant (OR 0.505; 95% CI, 0.356 to 0.716, fixed effect model) (Figure 8) (20,25,26,29,31,32,34,36,39,47,48,50,53,54).

Post-operative outcomes
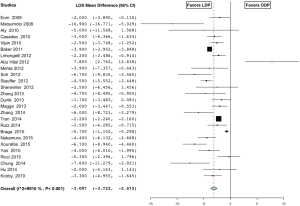
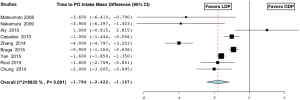
The length of stay in the LDP group was 9±4.4 days compared to 12±5.0 days in the ODP group and was significant (MD −3.097; 95% CI, −3.722 to −2.474) (Figure 9) (16-19,21,23,25-27,29-32,35,37,38,40,44-48,50,51,55). Return of bowel function occurred at a mean of 2.3±0.5 days in the LDP group compared to 3.7±0.5 days (MD −1.355; 95% CI, −2.051 to −0.660) (Figure 10) (23,25,26,30,38,49). Patients were able to tolerate oral intake in the LDP group at 3.3±1.7 vs. 5.2±1.5 days in the ODP group (MD −1.784; 95% CI, −2.422 to −1.147, I2=89.3%) (Figure 11) (19,23,25-27,30,32,38,49). Similarly, patients in the LDP group required fewer days of IV narcotics (MD −1.565; 95% CI, −2.251 to −0.678, P=0.001, fixed effect model) (Figure 12) (25,30,38) compared to the ODP group. There was a high level of heterogeneity among studies evaluating LOS (I2=99.1%), return of bowel function (I2=89.3%), and time to PO intake (I2=89.3%) and meta-regression analysis indicated that ASA of ≥3 (P<0.001) may be a significant explanation for some of the heterogeneity in these studies. The readmission rate in the LDP group was 12.3% versus 8.4% in the ODP group, which did not reach significance (OR 1.051; 95% CI, 0.494 to 2.234, P=0.898, I2=88.8%) (26,28,29,33-35,37,39,41,44,45,51,52,56). There was no difference in time to ambulation (LDP 1.5±0.5 vs. ODP 2.2±1.3) (MD −0.451; 95% CI, −0.958 to 0.056, P value 0.081, I2=87.2%) (25,30,32). There was a high level of heterogeneity among studies evaluating readmission rates (I2=88.8%) and time to ambulation (I2=87.2%). On meta-regression analysis, both age (P=0.008) and sample size (P=0.008) may explain heterogeneity significantly in the studies evaluating time to ambulation, and there was a trend with age (P=0.052) on readmission rates.


Laparoscopic pancreaticoduodenectomy
Selected studies
A total of 495 articles were reviewed, 19 of which were selected and included in the analysis. Fourteen articles were reviewed comparing MIPD to OPD. These articles included 24,457 patients (MIPD/OPD =3,510/20,947). Five articles were reviewed comparing RPD to OPD. The MIPD group included a total of 466 patients (RPD/OPD =182/184). No prospective randomized controlled trials were identified.
Patient selection
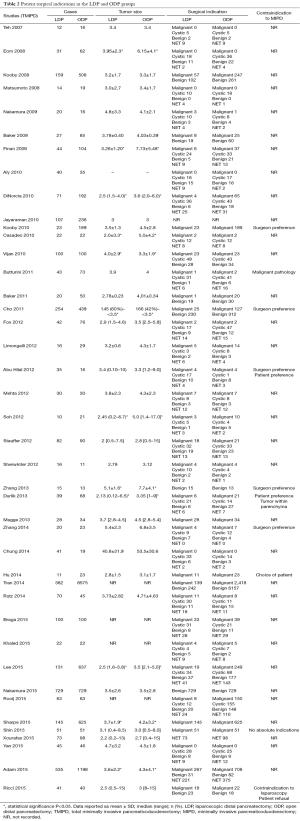
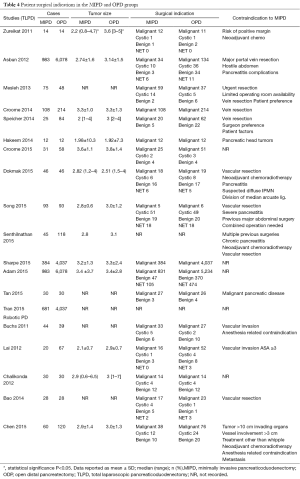
The mean age of patients in the MIPD group was 62.9±6.4 and 61.8±5.7 years in the OPD group. In the MIPD group, 54.0% (n=1,202) of patients were males and 51.4% (n=11,223) in the OPD group. In the MIPD group, 40.2% (n=126) of patients had an ASA of ≥3 and 48.7% (n=252) in the OPD group. The indication for operation was malignancy in 81.2% (n=1,653), benign/premalignant cystic disease in 5.4% (n=110), benign conditions in 6.7% (n=104), and NETs in 6.7% (n=137) in the MIPD group. The indication for operation was malignancy in 89.5% (n=10,035), benign/premalignant cystic disease in 1.2% (n=137), benign conditions in 4.7% (n=529), and NET in 4.6% (n=514) in the OPD group. The mean tumor size in the MIPD group was 2.89±0.56 and 3.08±0.51 cm. The most common contraindications to minimally invasive techniques reported in this review were neoadjuvant chemotherapy (59-62), hostile local conditions secondary to severe pancreatitis or previous complex abdominal operations (60,61,63,64), and need for potential vascular resection (59-61,63-70) (see Tables 3,4).
Full table
Full table
Intra-operative considerations
The mean operative time was MIPD was 470±58.9 minutes in the MIPD group and 375±84.9 minutes in the OPD group, which was significantly longer (MD 96.510; 95% CI, 56.622 to 136.397) (Figure 13) (59,63-66,68,69,71-73). There was a high level of heterogeneity (I2=94.8%) across studies and subsequent meta-regression analysis indicated that factors of tumor size (P<0.001) and ASA ≥3 (P=0.036) might be significant explanations for some of the heterogeneity. The reported estimated blood loss in the MIPD group was 542.4±353 and 911±497.8 cc in the OPD group (MD −351.083; 95% CI, −720.592 to 18.425) (60,63-66,71,72). There was a high level of heterogeneity (I2=98.7%) across studies and subsequent meta-regression analysis indicated that patient age (P<0.018), malignancy (P<0.001), and year (P=0.002) might be significant explanations for some of the heterogeneity. Despite similar blood loss, patients in the MIPD group required fewer red blood cell transfusions (OR 0.611; 95% CI, 0.422 to 0.884, fixed effect model) (Figure 14) (59,60,62,66,67). Conversion to an open operation was reported in 14 articles (59,60,62,63,65-74): 24.3% (n=332) of cases in the MIPD group and 6.0% (n=11) in the RPD group.


Oncologic outcomes
The mean number of LNs retrieved in the MIPD group was 17±4.9 and 16±4.7 LNs in the OPD group. In the analysis, the number of LNs retrieved was equivalent (MD 1.401; 95% CI, −0.468 to 3.271) (56,59,60,62-68,70,71,73,75,76). There was a high level of heterogeneity (I2=93.7%) across studies and subsequent meta-regression analysis indicated that ASA ≥3 (P<0.001) might explain some of the heterogeneity. Seventy percent of patients in the MIPD group had a positive LN compared to 66.4% in the OPD, which did not reach significance (OR 1.180; 95% CI, 0.969 to 1.435, fixed effect model) (56,67,76). The rate of positive margins was 17.3% in the MIPD group and 23.6% in the OPD group (OR 0.764; 95% CI, 0.607 to 0.962, fixed effect model) (Figure 15) (56,65,70,71,76).

Morbidity and mortality
The overall complication rate in the MIPD was 22.5% and 33.6% in the OPD group (OR 1.338; 95% CI, 0.905 to 1.978) (59,60,62-64,66,68,69,71-73,77). In-hospital or 30-day mortality was 3.9% in the MIPD group and 10.3% in the OPD group, which did not reach significance mortality (OR 1.091; 95% CI, 0.433 to 2.751) (56,59,60,62,64-66,68,70-73,75,77). Eight percent of patients in the MIPD group developed pancreatic fistulas compared to 3.1% in the OPD group (OR 0.948; 95% CI, 0.733 to 1.226, fixed effect model) (59,60,62-66,68-73,75). The rate of DGE was 3.4% in the MIPD group and 1.8% in the OPD group (OR 0.744; 95% CI, 0.527 to 1.050, fixed effect model) (59,60,63-69,71,73). The rate of bile leak was 1.0% in the MIPD group and 0.4% in the OPD group (OR 0.834; 95% CI, 0.411 to 1.695, fixed effect model) (59,60,63,66,68,73). Wound infections were similar occurring in 1.7% of patients in the MIPD group and 1.6% in the OPD group (OR 0.642; 95% CI, 0.404 to 1.021, fixed effect model) (59,63,65,66,68,70,71,75). Post-operative bleeding was more common in the MIPD group, 1.9% versus 0.5% (OR 2.028; 95% CI, 1.107 to 3.715, fixed effect model) (Figure 16) (59,60,63,66-68,71,73). There was a high level of heterogeneity across studies evaluating overall morbidity (I2=69.6%) and in-hospital mortality (I2=76.7%) and subsequent meta-regression analysis indicated that sample size (P=0.004) might explain some of the heterogeneity in mortality; however, no factors were found to be significant for overall morbidity.

Post-operative outcomes
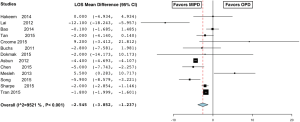
The mean length of stay in the MIPD group was 17±9.7 and 19±8.8 days in the OPD group, which was significantly shorter (MD −2.545; 95% CI, −3.852 to −1.237) (Figure 17) (56,59,60,63-66,68,69,72,73,75,77). There was a high level of heterogeneity across studies (I2=95.2%) and subsequent meta-regression analysis indicated that tumor size (P<0.001) and age (P<0.001) might explain some of the heterogeneity. Bowel function returned on average at 2.8±1.1 days in the MIPD group vs. 3.7±1.7 days in the OPD group, which was significantly quicker (MD −1.757; 95% CI, −2.025 to −1.488, fixed effect model) (Figure 18) (59,73). Similarly, patients in the MIPD group started PO intake at 4±1.3 days compared to 5.3±0.8 days in the OPD group (MD −1.423; 95% CI, −1.923 to −0.923), fixed effect model) (Figure 19) (59,64) than the open group. Patients in the MIPD group had similar reoperation rates (MIPD 2.3% vs. OPD 0.8%) (OR 0.958; 95% CI, 0.587 to 1.564, fixed effect model) (59,60,62,63,65,66,68-71) and readmission rates (MIPD 7.2% vs. OPD 9.1%) (OR 0.710; 95% CI, 0.497 to 1.014, fixed effect model) (56,59,60,64,65,70) as the open group.


Discussion
Laparoscopic pancreas surgery has been slow to evolve in comparison to other gastrointestinal surgery due to the intrinsic difficulty of operating on the pancreas and a steep learning curve involved in combining pancreas and laparoscopic surgical expertise. However, since the first descriptions of laparoscopic pancreaticoduodenectomy in 1994 and distal pancreatectomy in 1996 by Gagner and Pompe (12,13), minimally invasive pancreatectomies are being performed more frequently.
LDP has gained rapid acceptance and is associated with improved perioperative recovery, morbidity, mortality, and equivalent oncologic outcomes. In the largest single-center study to date, Song et al. evaluated 359 consecutive patients that underwent LDP for primarily benign disease and reported a median operative time of 195 minutes (range, 78–840 minutes), length of hospital stay of 8 days (range, 4–37 days), an overall complication rate of 12%, and a clinically significant pancreatic fistula occurring in 7% of patients (78). More recently, Sahakyan et al. performed a multicenter trial and analyzed postoperative and oncological outcomes in 196 patients with pancreatic adenocarcinoma undergoing LDP. In this study, operative time averaged 220 minutes, median length of stay was 8 days (range, 2–63 days), overall complications occurred in 31.9% of patients, and a clinically significant pancreatic fistula developed in 15.7% of patients. Additionally, 83.8% of patients had negative margins and median survival was 31.3 months (79). In this review, LDP was associated with longer operative times, reduced blood loss, lower rates of positive margins, shorter length of hospital stay, earlier return of bowel function, and a shorter time to oral intake in comparison to patients undergoing open distal pancreatectomy. As demonstrated by the heterogeneity and retrospective nature of these studies there is intrinsic bias when reviewing these data. However, as a community LDP is an accepted approach for the properly selected patients. At our institution, this approach is offered to all patients regardless of histology with relative contraindications, which include: comorbidities, BMI, tumor location, size and involvement of surrounding organs. However, these are not strict criteria and selection is based on individual surgeon and patient preference.
MIPD has been much slower to evolve, as the procedure is technically very demanding with multiple anastomosis and close proximity to major vasculature. However, in the National Cancer Database, MIPD was utilized in 14% of pancreaticoduodenectomies and its use increased by 45% from 402 cases in 2010 to 581 cases in 2011 (74). Patients undergoing MIPD are a highly select group of patients with favorable anatomical and disease factors. Adam et al. reported factors independently associated with undergoing MIPD and found that patients with fewer comorbidities, those being treated at an academic center, having a diagnosis of NET, and presenting at an earlier stage of disease all were associated with MIPD (74). The most common contraindications to MIPD reported in the literature include neoadjuvant chemotherapy (59-62), hostile local conditions secondary to severe pancreatitis or previous complex abdominal operations (60,61,63,64), and need for potential vascular resection (59-61,63-70). At our institution the primary contraindications MIPD are pancreatitis, neoadjuvant therapy, and vein resection. But we offer this approach to all resectable disease regardless of histology. Croome et al. reviewed 31 patients undergoing MIPD and OPD and concluded that MIPD with vascular resection achieves similar morbidity, mortality, and oncologic outcomes compared to patients undergoing OPD with major vascular resection (72).
It is well recognized that MIPD is a lengthy procedure due to the complexity of the operation, particularly during the early learning curve associated with MIPD. Gagner et al. first reported a mean operative time of 8.5 hours (range, 5.5–12 hours) in 1997 (80); however, as surgeons become more adept at MIPD, operative time has decreased significantly to 295 to 515 minutes (76,81-86) with a learning curve ranging from 10 cases (83,87,88) to 50 cases (64,70). We have found, at our institution, that operative time decreased from 366 minutes to 312 minutes after the first 15 cases (89), making the efficiency of the operation equivalent to the open operation in selected patients. In addition, a distinct advantage in this review was MIPD has reduced intraoperative blood loss ranging from 65 to 300 cc (76,81-85,89). We have found this to be consistent with our experience. This is likely secondary to patient selection, however, the superior views, the need for excellent hemostasis for visualization and magnification provided by minimally invasive techniques may also contribute to the reduction of blood loss.
Post-operative complications are common after MIPD and may occur in 29% to 42% (76,81,83,84) of patients. In particular, pancreatic fistula may occur in 7% to 25.8% of patients and is potentially life threatening (76,81,83,84,86). Patients with a soft pancreas and small pancreatic duct have a greater risk of pancreatic fistula (90) and there may be a selection bias for the incidence of fistula in MIPD as benign and early malignancy tends to be selected for this approach. In a matched analysis, Dokmak et al. reported a higher incidence of grade C pancreatic fistulas in the MIPD group and concluded that MIPD should only be considered in patients with a low risk of pancreatic fistula (60). In this review, patients in the MIPD group had similar overall complication rates and pancreatic fistula rates compared to OPD. In our experience with MIPD, although the majority of patients fit the criteria of a small duct and soft gland, the pancreatic grade C fistula rate was 7% and is comparable to OPD.
Prognostic factors that influence long-term outcome following PD include margin status, the number of nodes harvested, LN metastasis, grade of tumor differentiation, and vascular involvement (91,92). The accuracy of nodal staging is critically dependent on the number of LNs examined and 13 to 16 LNs are recommended (93). In the literature, LN yield in patients undergoing MIPD ranges from 7 to 18 LNs (76,80-82,84) and 0% to 11% of patients have positive margins (76,81,83,84). In this review, the mean number of LNs retrieved and margin status was similar between the open and minimally invasive techniques. Importantly, patients undergoing MIPD may be more likely to receive adjuvant chemotherapy (64,67). Additionally, minimally invasive surgery may offer distinct immunologic advantages in comparison to open operations including reduced stress of operation, attenuated impairment of the immune system, and reduced recurrence of malignancy (2,94,95).
Minimally invasive pancreatectomies may improve post-operative recovery. The length of hospital stay in patients undergoing MIPD ranges from 7 to 22 days (76,80,81,83-85) and return of bowel function has been reported to occur within 3.5 to 5.5 days (81,84). In this review, MIPD had a shorter length of hospital stay, earlier return of bowel function, and a shorter time to oral intake comparison to the open groups. We currently have ongoing quality of life studies to better understand the impact of MIPD.
There are significant limitations to the articles included in this review. In particular, there is a strong selection bias in selecting patients to undergo laparoscopic pancreatectomy versus open. Patients selected for laparoscopic techniques likely have differences in patient age, co-morbidities, tumor size, malignant features, BMI, vessel involvement, and history of abdominal operations that may result in a difficult dissection laparoscopically. These variables may lead to non-valid inferences in the outcomes associated with laparoscopic surgery. Further, the results in this review are likely due in part to publication bias in which studies that demonstrate negative findings such as an increase in morbidity and mortality in the laparoscopic group are likely to not be published. Additionally, there is a significant amount of heterogeneity in the studies included in this review. Although we attempted to identify differences in study parameters that may have led to this heterogeneity, it is a limitation inherent to systematic reviews and meta-analysis and the results must be interpreted with caution. Moreover, data published from high-volume institutions may be less generalizable to institutions that perform fewer minimally invasive cases.
Conclusions
In conclusion, this review and analysis of the available literature suggests that laparoscopic pancreatectomies are feasible, safe, reduce blood loss, improve perioperative recovery, and provide equivalent oncologic outcomes to open resection. The LDP experience is more mature than the laparoscopic pancreaticoduodenectomy experience. As experience increases there may be a change in other outcome endpoints. Even though it would be challenging with single institutional volumes, further investigation with collaborative randomized controlled trials is needed to avoid selection bias and control for confounding factors that are inherent to this type of analysis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (R. Charles Nichols Jr, Debashish Bose and George P. Kim) for the series “Pancreatic Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.12.02). The series “Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005;241:232-7. [PubMed]
- Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 2002;359:2224-9. [PubMed]
- Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477-84. [PubMed]
- Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [PubMed]
- Buunen M, Veldkamp R, Hop WC, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44-52. [PubMed]
- Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 2007;246:655-62; discussion 662-4. [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [PubMed]
- Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306-11. [PubMed]
- Tozzi R, Malur S, Koehler C, et al. Laparoscopy versus laparotomy in endometrial cancer: first analysis of survival of a randomized prospective study. J Minim Invasive Gynecol 2005;12:130-6. [PubMed]
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol 2012;30:695-700. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [PubMed]
- Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery 1996;120:1051-4. [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [PubMed]
- Dietz G, Dahabreh IJ, Gurevitch J, et al. OpenMEE: open-source, cross-platform software for ecological and evolutionary meta-analysis. [cited 2015 Sep 25]. Available online: http://www.cebm.brown.edu/openmee
- Abu Hilal M, Hamdan M, Di Fabio F, et al. Laparoscopic versus open distal pancreatectomy: a clinical and cost-effectiveness study. Surg Endosc 2012;26:1670-4. [PubMed]
- Durlik M, Matejak-Gorska M, Jaworowski R, et al. Laparoscopic distal pancreatectomy - new standard in the pancreatic surgery. Pol Przegl Chir 2013;85:589-97. [PubMed]
- Hu M, Zhao G, Wang F, et al. Laparoscopic versus open distal splenopancreatectomy for the treatment of pancreatic body and tail cancer: a retrospective, mid-term follow-up study at a single academic tertiary care institution. Surg Endosc 2014;28:2584-91. [PubMed]
- Ricci C, Casadei R, Taffurelli G, et al. Laparoscopic Distal Pancreatectomy in Benign or Premalignant Pancreatic Lesions: Is It Really More Cost-Effective than Open Approach? J Gastrointest Surg 2015;19:1415-24. [PubMed]
- Cho CS, Kooby DA, Schmidt CM, et al. Laparoscopic versus open left pancreatectomy: can preoperative factors indicate the safer technique? Ann Surg 2011;253:975-80. [PubMed]
- Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 2010;210:779-85, 786-7. [PubMed]
- Zhang RC, Yan JF, Xu XW, et al. Laparoscopic vs open distal pancreatectomy for solid pseudopapillary tumor of the pancreas. World J Gastroenterol 2013;19:6272-7. [PubMed]
- Zhang Y, Chen XM, Sun DL. Laparoscopic versus open distal pancreatectomy: a single-institution comparative study. World J Surg Oncol 2014;12:327. [PubMed]
- Butturini G, Partelli S, Crippa S, et al. Perioperative and long-term results after left pancreatectomy: a single-institution, non-randomized, comparative study between open and laparoscopic approach. Surg Endosc 2011;25:2871-8. [PubMed]
- Aly MY, Tsutsumi K, Nakamura M, et al. Comparative study of laparoscopic and open distal pancreatectomy. J Laparoendosc Adv Surg Tech A 2010;20:435-40. [PubMed]
- Braga M, Pecorelli N, Ferrari D, et al. Results of 100 consecutive laparoscopic distal pancreatectomies: postoperative outcome, cost-benefit analysis, and quality of life assessment. Surg Endosc 2015;29:1871-8. [PubMed]
- Casadei R, Ricci C, D'Ambra M, et al. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: a case-control study. Updates Surg 2010;62:171-4. [PubMed]
- Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9. [PubMed]
- Limongelli P, Belli A, Russo G, Cioffi L, et al. Laparoscopic and open surgical treatment of left-sided pancreatic lesions: clinical outcomes and cost-effectiveness analysis. Surg Endosc 2012;26:1830-6. [PubMed]
- Matsumoto T, Shibata K, Ohta M, et al. Laparoscopic distal pancreatectomy and open distal pancreatectomy: a nonrandomized comparative study. Surg Laparosc Endosc Percutan Tech 2008;18:340-3. [PubMed]
- Nakamura M, Wakabayashi G, Miyasaka Y, et al. Multicenter comparative study of laparoscopic and open distal pancreatectomy using propensity score-matching. J Hepatobiliary Pancreat Sci 2015;22:731-6. [PubMed]
- Yan JF, Kuang TT, Ji DY, et al. Laparoscopic versus open distal pancreatectomy for benign or premalignant pancreatic neoplasms: a two-center comparative study. J Zhejiang Univ Sci B 2015;16:573-9. [PubMed]
- DiNorcia J, Schrope BA, Lee MK, et al. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. J Gastrointest Surg 2010;14:1804-12. [PubMed]
- Finan KR, Cannon EE, Kim EJ, et al. Laparoscopic and open distal pancreatectomy: a comparison of outcomes. Am Surg 2009;75:671-9. [PubMed]
- Rutz DR, Squires MH, Maithel SK, et al. Cost comparison analysis of open versus laparoscopic distal pancreatectomy. HPB (Oxford) 2014;16:907-14. [PubMed]
- Teh SH, Tseng D, Sheppard BC. Laparoscopic and open distal pancreatic resection for benign pancreatic disease. J Gastrointest Surg 2007;11:1120-5. [PubMed]
- Baker MS, Bentrem DJ, Ujiki MB, et al. Adding days spent in readmission to the initial postoperative length of stay limits the perceived benefit of laparoscopic distal pancreatectomy when compared with open distal pancreatectomy. Am J Surg 2011;201:295-9; discussion 299-300. [PubMed]
- Chung JC, Kim HC, Song OP. Laparoscopic distal pancreatectomy for benign or borderline malignant pancreatic tumors. Turk J Gastroenterol 2014;25:162-6. [PubMed]
- de Rooij T, Jilesen AP, Boerma D, et al. A nationwide comparison of laparoscopic and open distal pancreatectomy for benign and malignant disease. J Am Coll Surg 2015;220:263-70.e1.
- Eom BW, Jang JY, Lee SE, et al. Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surg Endosc 2008;22:1334-8. [PubMed]
- Fox AM, Pitzul K, Bhojani F, et al. Comparison of outcomes and costs between laparoscopic distal pancreatectomy and open resection at a single center. Surg Endosc 2012;26:1220-30. [PubMed]
- Khaled YS, Malde DJ, Packer J, et al. A Case-matched Comparative Study of Laparoscopic Versus Open Distal Pancreatectomy. Surg Laparosc Endosc Percutan Tech 2015;25:363-7. [PubMed]
- Lee SY, Allen PJ, Sadot E, et al. Distal pancreatectomy: a single institution's experience in open, laparoscopic, and robotic approaches. J Am Coll Surg 2015;220:18-27. [PubMed]
- Magge D, Gooding W, Choudry H, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg 2013;148:525-31. [PubMed]
- Mehta SS, Doumane G, Mura T, et al. Laparoscopic versus open distal pancreatectomy: a single-institution case-control study. Surg Endosc 2012;26:402-7. [PubMed]
- Sherwinter DA, Lewis J, Hidalgo JE, et al. Laparoscopic distal pancreatectomy. JSLS 2012;16:549-51. [PubMed]
- Soh YF, Kow AW, Wong KY, et al. Perioperative outcomes of laparoscopic and open distal pancreatectomy: our institution's 5-year experience. Asian J Surg 2012;35:29-36. [PubMed]
- Stauffer JA, Rosales-Velderrain A, Goldberg RF, et al. Comparison of open with laparoscopic distal pancreatectomy: a single institution's transition over a 7-year period. HPB (Oxford) 2013;15:149-55. [PubMed]
- Nakamura Y, Uchida E, Aimoto T, et al. Clinical outcome of laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Surg 2009;16:35-41. [PubMed]
- Tran Cao HS, Lopez N, Chang DC, et al. Improved perioperative outcomes with minimally invasive distal pancreatectomy: results from a population-based analysis. JAMA Surg 2014;149:237-43. [PubMed]
- Xourafas D, Tavakkoli A, Clancy TE, et al. Distal pancreatic resection for neuroendocrine tumors: is laparoscopic really better than open? J Gastrointest Surg 2015;19:831-40. [PubMed]
- Adam MA, Choudhury K, Goffredo P, et al. Minimally Invasive Distal Pancreatectomy for Cancer: Short-Term Oncologic Outcomes in 1,733 Patients. World J Surg 2015;39:2564-72. [PubMed]
- Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg 2008;248:438-46. [PubMed]
- Velanovich V. Case-control comparison of laparoscopic versus open distal pancreatectomy. J Gastrointest Surg 2006;10:95-8. [PubMed]
- Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg 2010;145:616-21. [PubMed]
- Sharpe SM, Talamonti MS, Wang CE, et al. Early National Experience with Laparoscopic Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Comparison of Laparoscopic Pancreaticoduodenectomy and Open Pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg 2015;221:175-84. [PubMed]
- Sharpe SM, Talamonti MS, Wang E, et al. The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. Am J Surg 2015;209:557-63. [PubMed]
- Baker MS, Bentrem DJ, Ujiki MB, et al. A prospective single institution comparison of peri-operative outcomes for laparoscopic and open distal pancreatectomy. Surgery 2009;146:635-43; discussion 643-5. [PubMed]
- Chen S, Chen JZ, Zhan Q, et al. Robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc 2015;29:3698-711. [PubMed]
- Dokmak S, Fteriche FS, Aussilhou B, et al. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg 2015;220:831-8. [PubMed]
- Senthilnathan P, Chinnusamy P, Ramanujam A, et al. Comparison of Pathological Radicality between Open and Laparoscopic Pancreaticoduodenectomy in a Tertiary Centre. Indian J Surg Oncol 2015;6:20-5. [PubMed]
- Zureikat AH, Breaux JA, Steel JL, et al. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg 2011;15:1151-7. [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [PubMed]
- Song KB, Kim SC, Hwang DW, et al. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg 2015;262:146-55. [PubMed]
- Bao PQ, Mazirka PO, Watkins KT. Retrospective comparison of robot-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg 2014;18:682-9. [PubMed]
- Buchs NC, Addeo P, Bianco FM, et al. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg 2011;35:2739-46. [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633-8; discussion 638-40. [PubMed]
- Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy--a comparative study. Int J Surg 2012;10:475-9. [PubMed]
- Mesleh MG, Stauffer JA, Bowers SP, et al. Cost analysis of open and laparoscopic pancreaticoduodenectomy: a single institution comparison. Surg Endosc 2013;27:4518-23. [PubMed]
- Speicher PJ, Nussbaum DP, White RR, et al. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol 2014;21:4014-9. [PubMed]
- Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc 2012;26:2397-402. [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg 2015;19:189-94. [PubMed]
- Tan CL, Zhang H, Peng B, et al. Outcome and costs of laparoscopic pancreaticoduodenectomy during the initial learning curve vs laparotomy. World J Gastroenterol 2015;21:5311-9. [PubMed]
- Adam MA, Roman SA, Sosa JA. Minimally Invasive Versus Open Pancreaticoduodenectomy for Cancer Is Associated With Increased 30-Day Mortality. Ann Surg 2015; [Epub ahead of print]. [PubMed]
- Hakeem AR, Verbeke CS, Cairns A, et al. A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: oncological outcomes using Leeds Pathology Protocol. Hepatobiliary Pancreat Dis Int 2014;13:435-41. [PubMed]
- Senthilnathan P, Srivatsan Gurumurthy S, Gul SI, et al. Long-term results of laparoscopic pancreaticoduodenectomy for pancreatic and periampullary cancer-experience of 130 cases from a tertiary-care center in South India. J Laparoendosc Adv Surg Tech A 2015;25:295-300. [PubMed]
- Tran TB, Dua MM, Worhunsky DJ, et al. The First Decade of Laparoscopic Pancreaticoduodenectomy in the United States: Costs and Outcomes Using the Nationwide Inpatient Sample. Surg Endosc 2015; [Epub ahead of print]. [PubMed]
- Song KB, Kim SC, Park JB, et al. Single-center experience of laparoscopic left pancreatic resection in 359 consecutive patients: changing the surgical paradigm of left pancreatic resection. Surg Endosc 2011;25:3364-72. [PubMed]
- Sahakyan MA, Kazaryan AM, Rawashdeh M, et al. Laparoscopic distal pancreatectomy for pancreatic ductal adenocarcinoma: results of a multicenter cohort study on 196 patients. Surg Endosc 2015; [Epub ahead of print]. [PubMed]
- Gagner M, Pomp A. Laparoscopic pancreatic resection: Is it worthwhile? J Gastrointest Surg 1997;1:20-5; discussion 25-6. [PubMed]
- Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc 2006;20:1045-50. [PubMed]
- Gumbs AA, Gayet B. The laparoscopic duodenopancreatectomy: the posterior approach. Surg Endosc 2008;22:539-40. [PubMed]
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. [PubMed]
- Palanivelu C, Jani K, Senthilnathan P, et al. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg 2007;205:222-30. [PubMed]
- Pugliese R, Scandroglio I, Sansonna F, et al. Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech 2008;18:13-8. [PubMed]
- Wang M, Zhang H, Wu Z, et al. Laparoscopic pancreaticoduodenectomy: single-surgeon experience. Surg Endosc 2015;29:3783-94. [PubMed]
- Wang Y, Bergman S, Piedimonte S, et al. Bridging the gap between open and minimally invasive pancreaticoduodenectomy: the hybrid approach. Can J Surg 2014;57:263-70. [PubMed]
- Kuroki T, Kitasato A, Adachi T, et al. Learning Curve for Laparoscopic Pancreaticoduodenectomy: A Single Surgeon's Experience with Consecutive Patients. Hepatogastroenterology 2014;61:838-41. [PubMed]
- Paniccia A, Schulick RD, Edil BH. Total Laparoscopic Pancreaticoduodenectomy: A Single-Institutional Experience. Ann Surg Oncol 2015;22:4380-1. [PubMed]
- Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 2008;32:419-28. [PubMed]
- Westgaard A, Tafjord S, Farstad IN, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer 2008;8:170. [PubMed]
- Qiao QL, Zhao YG, Ye ML, et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg 2007;31:137-43; discussion 144-6. [PubMed]
- Valsangkar NP, Bush DM, Michaelson JS, et al. N0/N1, PNL, or LNR? The effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointest Surg 2013;17:257-66. [PubMed]
- Hartley JE, Mehigan BJ, Monson JR. Alterations in the immune system and tumor growth in laparoscopy. Surg Endosc 2001;15:305-13. [PubMed]
- Whitson BA, D'Cunha J, Maddaus MA. Minimally invasive cancer surgery improves patient survival rates through less perioperative immunosuppression. Med Hypotheses 2007;68:1328-32. [PubMed]


