
“Boom-Boom” radioimmunotherapy of lymphomas: are two magic bullets better than one?
Since its first human use, dated back to 1987 (1), radioimmunotherapy (RIT) of non-Hodgkin (NHL) lymphomas has undergone many vicissitudes, and we might be not too far from the truth by affirming that this is not the best time for it (2). As a matter of fact, the worldwide enthusiasm following the approval, at the beginning of this century, of the first two RIT compounds, 90Y-ibritumomab-tiuxetan (Zevalin®) and 131I-tositumomab (Bexxar®) for relapsed/refractory indolent NHL, was tempered by the success of new chemotherapy agents (3) and of the rituximab maintenance strategies (4). Moreover, in case of relapse after optimized rituximab-including treatments, RIT showed a reduced efficacy both in aggressive and indolent NHLs (5,6). In addition, the absence of randomized phase III studies comparing RIT head-to-head with other agents and the physicians’ natural reluctance to refer patients to radionuclide treatments, have played in synergy against its use. At present, RIT is underused (Figure 1) and, in February 2014, this has led to the withdrawal of Bexxar® from the US market.
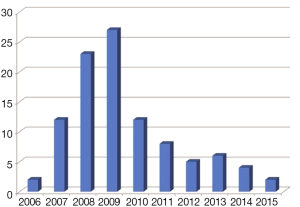
Yet, RIT is by far the most effective and least toxic single treatment for NHL, and it is largely preferred by patients over other therapeutic options (7-9); in fact, none of the available anti-cancer agents would be able to produce as high as 87% ORR (including 56% CR/Cru) or 95% ORR (including 75% CR/Cru) after a single infusion, as obtained with frontline Zevalin® or Bexxar®, respectively (7,8).
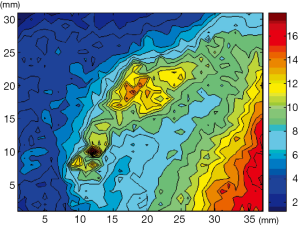
Interestingly, nuclear medicine has the intrinsic potential of allowing pre and post-therapeutic in-vivo biodistribution studies, which might inform the therapeutic infusion of RIT. By applying computational analysis on radioactivity distribution in organs or tumor lesions over time, internal dosimetry allows for obtaining dose calculations in these body compartments (Figure 2). Indeed, doses to organs and target lesions can vary intra and inter-patients because of the influence of all possible physical and biological variables in RIT, such as scheme of RIT fractionation, amount of antibody preloading, changes in size and biology etc. Unfortunately, such dosimetry studies are seldom accomplished in clinical practice, thought they might help to evaluate the effect of all these variables on RIT efficacy and toxicity, pursuing patient-specific treatment optimization.
Planar dosimety using a tracing amount of 131I-labeled antibody is part of the standard protocol for Bexxar® infusion, where the therapeutic administered activity is planned on a single-patient basis in order to keep the resulting total-body dose (TBD) within a predetermined limit (i.e., <75 cGy or <65 cGy in patients with platelet counts ≥ or ≤150,000/mL, respectively). As a result, the range of administered therapeutic activity per single patient is wide, that is between 47 and 212 mCi (1.74–7.8 GBq), median 91 mCi (3.36 GBq) (10). Interestingly, a significantly longer duration of response was shown for patients receiving higher TBD (>65 cGy) if compared to patients receiving less than 55 cGy (11).
Conversely, the activity to be administered in RIT with the radiometal conjugate 90Y-ibritumomab-tiuxetan takes into account patient weight and platelet blood count only, and no optimization based on pre-therapeutic dosimetry is considered.
The choice of avoiding dosimetry in case of Zevalin® has several reasons. First, the biodistribution of radio-metal conjugates is generally thought to be better predictable than that of radio-halogens. Second, dosimetry of 90Y-ibritumomab is complicated by the technical impossibility of obtaining γ-camera images by means of the pure β-emitter 90Y, which requires the labeling of ibritumomab with a γ-emitting surrogate, such as 111In. Third, and probably of greater importance, the marketing of RIT compounds has preceded many recent technical and theoretical achievements of internal dosimetry which, when RIT was developed, was just not advanced enough to match clinical needs and expectations. In fact, radiobiological modeling has only recently been applied to radionuclide treatments and is continuously evolving as new questions arise from therapies implying different physical and biological effects (12-14). In addition, only the breakthrough of hybrid SPECT/CT cameras has allowed accounting for errors and spatial heterogeneities in dose calculations, facilitating patient-specific voxel-based dosimetry and implementing radiobiological modeling (15). As such, standard planar dosimetry is no longer a good model for optimizing RIT efficacy, and three-dimensional, voxel-based dosimetry is warranted. Only recently three dimensional dosimetry and radiobiological modeling have been applied to RIT: a few reports have been published supporting a dose-response relationship for NHL nodal lesions treated with Bexxar® (16), while tumor voxel-based dosimetry of Zevalin® is still at its beginning (17,18).
In synthesis, much room does still exist for improvement of RIT efficacy and optimization of delivery and the feeling is that RIT is not only underprescribed but also underdosed.
An excellent effort toward dose optimization in RIT is represented by a recently published paper from a cooperative international research group reporting on the efficacy and toxicity of 90Y-ibritumomab-tiuxetan delivered in two fractions as frontline therapy in patients with follicular lymphoma (FL) (19). From the 76 recruited, a total of 72 patients entered the final protocol; fifty-five patients (76%) received both infusions. Eight and four patients did not proceed with the second RIT infusion because of bone marrow toxicity (BM) and treating physician’s discretion, respectively. Additionally, 4 patients developed mouse antibodies (HAMA) after the first cycle and one patient did not undergo the second infusion for underlining psychiatric disease. Most patients (78%) were stage III/IV; 44% patients had high-risk FLIPI. Patients with more that 20% BM infiltration were pretreated with four weekly infusions of rituximab 375 mg/m2 and entered the study provided that <20% BM infiltration was achieved.
RIT infusions were administered 8 weeks apart, unless otherwise indicated by slow BM recovery. 90Y-ibritumomab-tiuxetan was given at 11.1 MBq/Kg and injected activities were capped at 888 MBq (24 mCi). Such protocol showed an excellent 95.8% ORR including 69.4% CR/Cru, and a projected 3-year PFS of 58%. Interestingly, in contrast to previous observations, there was no significant difference in PFS between patients with tumor size < or >5 cm (65.4% vs. 50.2%, P=0.47). Hematological toxicity profile was acceptable: grade 4 thrombocytopenia and neutrophenia occurred in 6.9% and 8.3% of patients after the first infusion, increasing to 21.8% and 14.5% after the second infusion, respectively. After the second RIT, 8 (14.5%) patients received platelets and the same number of patients received red cell transfusions. Two (2.8%) neutropenic sepses were observed in the entire cohort. It is worth reminding, however, that 8 patients (11% of the initial cohort) could not undergo the second RIT infusion because of prolonged BM suppression after the first treatment.
Dose fractionation has a strong theoretical rationale both in external beam radiation therapy (EBRT) and in RIT since, according to the classical linear-quadratic model, it makes possible to increase the total dose delivered to tumor by decreasing normal tissue toxicity. An additional advantage of dose fractionation in RIT would be the possibility to achieve more uniform dose distributions within tumors by progressively reducing tumor size and improving blood supply (20). However, the same radiobiological principles do not necessarily apply identically to both EBRT and RIT, as the latter involves heterogeneous, continuous and continuously decreasing low-dose-rate radiation, which effects on cell killing have yet to be fully understood (21). As a matter of fact, it is interesting to note that non-targeted effects, including apoptosis, mutations, cell transformation, release of stress signals, are probably prevalent in RIT as they occur after low dose or low dose-rate irradiation (21). These so called “bystander” effects might not be fitted by linear or linear-quadratic models, rather they might saturate after a certain dose threshold, questioning the superior efficacy of the dose-fractionation vs. standard, single treatment approach in RIT, which indeed has yet to be experimentally determined in patients (21). In addition, there are other non-radiation dependent immunological effects of RIT which might help to explain the excellent response of some tumor to very low radiation burden. For example, it has been suggested that the benefit of RIT would be higher in patients with preserved T cell immunity, which might complement the effect of radiation by eliciting a cell-mediated toxicity against the mouse monoclonal antibodies used in RIT (22).
Some responses to the radiobiological questions regarding efficacy of dose fractionation on tumor control in RIT might come from the study of Illidge et al. (19). A retrospective dosimetric analysis of 28 patients from this cohort revealed that organ absorbed doses were similar for both fractions and that an image-based, 3D method for BM dosimetry was predictive of hematological toxicity (23). Unfortunately, however, at the time of writing no data have yet been published on the results of tumor dosimetry in these patients.
Given its complexity and all the reasons we briefly outlined above, not surprisingly dosimetry was only retrospectively analyzed and not used to inform treatment schedule in this trial. Therefore, important radiobiologic and immunologic questions still need to be addressed. Nonetheless, the study of Illidge and colleagues is encouraging and could potentially pave the way for the conception and design of future trials aiming at a radiobiological optimization of RIT delivery. With particular regard to tumor dose-effect relationships, there might be a bulk of relevant information arising from combined dosimetric, clinical and laboratory data of this study, which would be otherwise lost if not fully analyzed and discussed. In other words, this study might have still a lot to say on the effects of RIT.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.12.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeNardo SJ, DeNardo GL, O'Grady LF, et al. Treatment of a patient with B cell lymphoma by I-131 LYM-1 monoclonal antibodies. Int J Biol Markers 1987;2:49-53. [PubMed]
- Reagan PM, Friedberg JW. Advancing radioimmunotherapy and its future role in non-Hodgkin lymphoma. Future Oncol 2015;11:1543-53. [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [PubMed]
- Press OW, Unger JM, Rimsza LM, et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol 2013;31:314-20. [PubMed]
- Morschhauser F, Illidge T, Huglo D, et al. Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood 2007;110:54-8. [PubMed]
- Cicone F, Russo E, Carpaneto A, et al. Follicular lymphoma at relapse after rituximab containing regimens: comparison of time to event intervals prior to and after 90Y-ibritumomab-tiuxetan. Hematol Oncol 2011;29:131-8. [PubMed]
- Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med 2005;352:441-9. [PubMed]
- Scholz CW, Pinto A, Linkesch W, et al. (90)Yttrium-ibritumomab-tiuxetan as first-line treatment for follicular lymphoma: 30 months of follow-up data from an international multicenter phase II clinical trial. J Clin Oncol 2013;31:308-13. [PubMed]
- Federico M, Luminari S, Dondi A, et al. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol 2013;31:1506-13. [PubMed]
- Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol 2001;19:3918-28. [PubMed]
- Wahl RL, Zasadny KR, MacFarlane D, et al. Iodine-131 anti-B1 antibody for B-cell lymphoma: an update on the Michigan Phase I experience. J Nucl Med 1998;39:21S-27S. [PubMed]
- Pouget JP, Navarro-Teulon I, Bardiès M, et al. Clinical radioimmunotherapy--the role of radiobiology. Nat Rev Clin Oncol 2011;8:720-34. [PubMed]
- Pacilio M, Betti M, Cicone F, et al. A theoretical dose-escalation study based on biological effective dose in radioimmunotherapy with (90)Y-ibritumomab tiuxetan (Zevalin). Eur J Nucl Med Mol Imaging 2010;37:862-73. [PubMed]
- Hobbs RF, Howell RW, Song H, et al. Redefining relative biological effectiveness in the context of the EQDX formalism: implications for alpha-particle emitter therapy. Radiat Res 2014;181:90-8. [PubMed]
- Dewaraja YK, Frey EC, Sgouros G, et al. MIRD pamphlet No. 23: quantitative SPECT for patient-specific 3-dimensional dosimetry in internal radionuclide therapy. J Nucl Med 2012;53:1310-25. [PubMed]
- Dewaraja YK, Schipper MJ, Shen J, et al. Tumor-Absorbed Dose Predicts Progression-Free Survival Following (131)I-Tositumomab Radioimmunotherapy. J Nucl Med 2014;55:1047-53. [PubMed]
- D'Arienzo M, Cicone F, Chiacchiararelli L, et al. Three-dimensional patient-specific dosimetry in radioimmunotherapy with 90Y-ibritumomab-tiuxetan. Cancer Biother Radiopharm 2012;27:124-33. [PubMed]
- Cicone F, D'Arienzo M, Carpaneto A, et al. Quantification of dose nonuniformities by voxel-based dosimetry in patients receiving 90Y-ibritumomab-tiuxetan. Cancer Biother Radiopharm 2013;28:98-107. [PubMed]
- Illidge TM, Mayes S, Pettengell R, et al. Fractionated 90Y-ibritumomab tiuxetan radioimmunotherapy as an initial therapy of follicular lymphoma: an international phase II study in patients requiring treatment according to GELF/BNLI criteria. J Clin Oncol 2014;32:212-8. [PubMed]
- DeNardo GL, Schlom J, Buchsbaum DJ, et al. Rationales, evidence, and design considerations for fractionated radioimmunotherapy. Cancer 2002;94:1332-48. [PubMed]
- Pouget JP, Lozza C, Deshayes E, et al. Introduction to radiobiology of targeted radionuclide therapy. Front Med (Lausanne) 2015;2:12. [PubMed]
- Buchegger F, Mach JP, Press OW, et al. Improving the chance of cure of follicular lymphoma by combining immunotherapy and radioimmunotherapy based on anti-CD20 antibodies? Ann Oncol 2013;24:1948-9. [PubMed]
- Ferrer L, Malek E, Bodet-Milin C, et al. Comparisons of dosimetric approaches for fractionated radioimmunotherapy of non-Hodgkin lymphoma. Q J Nucl Med Mol Imaging 2012;56:529-37. [PubMed]


