
A bibliometric analysis of CD38-targeting antibody therapy in multiple myeloma from 1985 to 2021
Introduction
Multiple myeloma (MM) is a monoclonal plasma cell malignant disease in which the secretion of monoclonal immunoglobulins can be found in the serum or urine (1). Proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs) and traditional drugs such as glucocorticoids are widely accepted treatment options. However, inherent or acquired drug tolerance results in poor prognosis in the long term and has encouraged researchers to search for new drugs (2). While CD38 is a transmembrane glycoprotein that is relatively highly expressed on MM cells (3-5), it activates the invention and development of CD38-targeting antibodies such as daratumumab (DARA) to treat MM (6-8). CD38-targeting antibodies have pleiotropic mechanisms, including killing tumor cells via Fc-dependent immune effectors, immunomodulatory activity and apoptotic effects (9-12). According to clinical studies, CD38-targeting antibodies have a significant curative effect on newly diagnosed/relapsed and refractory MM (RRMM) patients alone or in combination (8,13,14). There is an urgent need to develop antibody therapeutics for hematologic malignancies such as MM. The therapeutic potential of CD38-targeting antibodies has emerged before our eyes. These antibodies show tremendous potential for treating other hematologic malignancies via antibody-dependent cellular cytotoxicity (ADCC) or phagocytosis (ADCP) (9,11). As there are an increasing number of studies concerning CD38-targeting antibody therapy in MM and other hematological malignancies, it is necessary to make a brief introduction and summarization. It is of great importance to review the academic progress of CD38-targeting antibody therapy in MM to discover not only new applications of CD38-targeting antibody therapy but also progress in the treatment of MM.
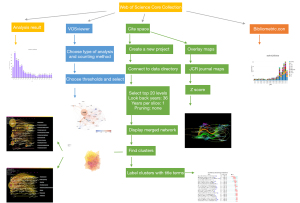
Bibliometric is an established methodology to analyze and visualize the further identifying important issues in research field and connection between authors, institutions and countries using software such as VOSviewer (15), HistCite and CiteSpace (16). These tools were used to analyze all the relevant articles from 1985 to June 21, 2021, and the results, including the key contributors of this research, the milestones of the field, the progress of the research hotspots and the prediction of future forefronts, are presented in the form of vivid graphs. This article, aiming to analyze the literature concerning CD38-targeting antibody therapy in MM to identify its course of development and structural relationships in this research field, is the first comprehensive article on immunological therapy in MM.
Methods
Data collection and extraction
Relevant literatures were retrieved from the Web of Science Core Collection (WoSCC) database on June 21, 2021. The keywords “multiple myeloma” and “CD38” were used to extract publications published between 1985 and June 21, 2021. Then, we exported full records and cited references in the form of plain text and tab-delimited (Win, UTF-8) for the use of scientometric implements.
Statistical analysis
In total, 1,030 articles were exploited from the WoSCC. Three types of bibliometric implements, CiteSpace (Chaomei Chen, Drexel University, USA), VOSviewer (Nees Jan van Eck and Ludo Waltman, Leiden University of Centre for Science and Technology Studies, Netherlands) and bibliometric.com, were used to analyze the bibliometric indicators relating to authors, institutes and countries/regions.
CiteSpace (Version 5.7.R5 64-bit), a free Java-based software, was used to visualize and identify hot spots of the research field by mapping authors/references/countries with nodes and links, exploiting highly cited keywords and analyzing the time trend of keywords. According to the definition of CiteSpace, every node indicates an author/reference/country, while the links between nodes indicate a cowork or cocitation (17). Cocitation demonstrates the frequency of jointly cited documents. Every node represents a cited article, and every connecting line represents a cocitation project. This tool can be used not only to identify the relationships among studies but also to visualize highly recognized relationships. To reveal the knowledge evolution in the topic of “CD38-targeting antibody therapy in MM”, we utilized cluster analysis, set visualization in the form of a timeline or timezone view, analyzed burstness and generated a dual-map. Cluster analysis is a statistical technique employed to identify the structure of the literature, in which studies are separated into different clusters. In addition, different clusters appear to be dissimilar. Similarity and dissimilarity are represented by the distance between clusters. A timeline view is used to visualize the variation tendency of every keyword in clusters. Timezone view is used to identify the emerging keywords over time. Every circle implies a keyword, and its size indicates the frequency of appearance. The lines between circles imply co-occurrence. Burst detection serves to display the strength tendency of a phrase over time. Dual-map overlay is a portfolio analysis, ranging from authors/institutes/countries, term clustering and quotation systems, to inform researchers about the position of the object of interest.
VOSviewer (Version 1.6.16.0) is also free-access Java-based software that helps in determining not only the coauthorship and cocitation relationships among authors, journals and countries/regions but also the co-occurrence of the keywords. Networks are also constructed to collect information on the top authors, journals, institutions and countries. Network visualization focuses on the distribution of hotspots, the relationship between research individuals/institutes/countries and its strength. Overlay visualization places emphasis on the annual change, while density visualization emphasizes the density of occurrence.
bibliometric.com is an online platform for publication analysis collected by China Science Digital Library. It is used for tracking advanced countries/regions in the research field over the years.
Results
Annual publications and trend
The data processing procedure is shown in the flow diagram (Figure 1). A total of 1,030 articles from the WoSCC were searched. The number of articles meeting the search criteria remained at a steady and relatively low level from 1999 to 2014, increased rapidly from 2015 and reached a three-digit number after 2018 (Figure 2A). All these articles were cited 24,332 times in total, with an average of 23.62 times.
Contribution of countries/regions and institutions
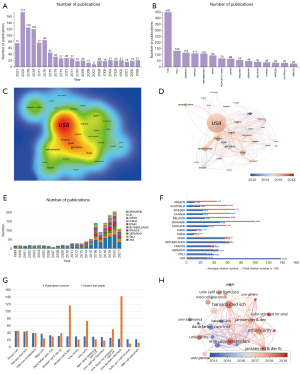
The countries/regions published a total of 57 articles on CD38 and MM. Researchers from diverse countries/regions also collaborated on some of the articles. A total of 1,433 publications were produced by the top 15 countries/regions with the highest number of publications. The USA, with 450 publications, far outweighed other countries/regions regarding the number of publications [Italy (n=133), Germany (n=118), France (n=111), the Netherlands (n=108), Spain (n=94), China (n=70), Japan (n=68), England (n=56) and Denmark (n=49)] (Figure 2B). The same conclusion could be obtained through the heatmap created by VOSviewer. The USA shared the highest production density, followed by Italy, Germany and France (Figure 2C). The burst of article publication differed from country to country. As Japan and Spain principally published articles before 2015, the USA, Italy, France and the Netherlands had a burst of publication from 2015 to 2021 (Figure 2D). To determine the variation trend of publication proportions in the top 10 productive countries/regions, we used bibliometric.com and obtained relevant results affirming the former conclusions (Figure 2E).
The number of citations per paper is crucial for analyzing the research quality and contribution of countries/regions on CD38-targeting antibody therapy in MM, while the citations between countries/regions can demonstrate the collaborations between countries/regions. We analyzed the collected data from the WoSCC and found that the USA (n=13,823), the Netherlands (n=6,310) and Spain (n=5,656) were the top three countries with the highest total citation numbers, while the USA far exceeded other countries/regions. However, countries such as Denmark (n=99.96) had the highest citation frequency (Figure 2F), suggesting the significant breakthrough and high-quality articles were derived from it.
All 1,635 institutions were interrelated in this field. The top 15 productive institutions were graphed and visualized with their total publication number and citations per paper (Figure 2G). Emory University (n=45) ranked first, followed by Harvard Medical School (n=43) and Vrije University Amsterdam (n=39). Most studies were published after 2017 (Figure 2H). Among these 15 institutions, 11 were assigned to the USA, 2 to Italy, and the rest to Denmark and the Netherlands (Table 1). Among them, Genmab, an international biotech company, had the highest number of citations per publication (170 times per paper).
Table 1
| Rank | Institution | Country/region | Citations | Publications | Average citation per paper |
|---|---|---|---|---|---|
| 1 | Janssen Res & Dev | USA | 3,485 | 30 | 116.17 |
| 2 | Univ Southern Denmark | Denmark | 3,264 | 23 | 141.91 |
| 3 | Genmab | Denmark | 2,890 | 17 | 170.00 |
| 4 | Vejle Hosp | Denmark | 2,566 | 20 | 128.30 |
| 5 | Univ Turin | Italy | 2,179 | 30 | 72.63 |
| 6 | Emory Univ | USA | 2,054 | 45 | 45.64 |
| 7 | Harvard Univ | USA | 1,963 | 20 | 98.15 |
| 8 | Harvard Med Sch | USA | 1,950 | 43 | 45.34 |
| 9 | Univ Med Ctr Utrecht | Netherlands | 1,661 | 21 | 79.10 |
| 10 | Vrije Univ Amsterdam | Netherlands | 1,530 | 39 | 39.23 |
| 11 | Univ Salamanca | Spain | 1,464 | 20 | 73.20 |
| 12 | Univ Texas Md Anderson Canc Ctr | USA | 1,291 | 26 | 49.65 |
| 13 | Janssen Res & Dev Llc | USA | 1,194 | 31 | 38.52 |
| 14 | Univ Penn | USA | 1,173 | 15 | 78.20 |
| 15 | Univ Athens | Greece | 987 | 15 | 65.80 |
Publication distribution among journals
All 1,030 publications were included in 286 Science Citation Index-Expanded (SCI-E) recorded journals. In terms of the number of publications, Blood (n=109), Clinical Lymphoma Myeloma and Leukemia (n=40) and Haematologica (n=33) were top 3 journals. The 438 articles published by the top 20 most productive journals accounted for 42.5 percent of the total. Among these journals, 8 were assigned to the USA, while 7 were assigned to England. It is obvious that Blood had the highest number of total citations (n=4,711), and New England Journal of Medicine had the highest number of citations per paper (n=361.86). In addition, the top 3 journals ranked by the 2020 impact factor were Journal of Clinical Oncology (32.956), Blood (17.543) and Clinical Cancer Research (10.107) (Table 2).
Table 2
| Rank | Journal name | Country/region | Citations | Average citation per paper | Journal impact factor of 2020 |
|---|---|---|---|---|---|
| 1 | Blood | USA | 4,711 | 43.22 | 17.543 |
| 2 | New England Journal of Medicine | USA | 2,533 | 361.86 | 74.699 |
| 3 | Leukemia | England | 1,222 | 39.42 | 8.665 |
| 4 | Clinical Cancer Research | USA | 1,017 | 46.23 | 10.107 |
| 5 | Journal of Immunology | USA | 875 | 109.38 | 4.886 |
| 6 | Haematologica | Italy | 694 | 21.03 | 7.116 |
| 7 | British Journal of Haematology | England | 673 | 21.71 | 5.518 |
| 8 | Haematologica-the Hematology Journal | Italy | 663 | 73.67 | |
| 9 | Cancer Research | USA | 662 | 30.09 | 9.727 |
| 10 | Frontiers in Immunology | Switzerland | 570 | 22.80 | 5.085 |
| 11 | Cytometry Part B-Clinical Cytometry | USA | 420 | 16.80 | 2.070 |
| 12 | American Journal of Clinical Pathology | USA | 400 | 50.00 | 2.094 |
| 13 | Transfusion | USA | 385 | 24.06 | 2.800 |
| 14 | Leukemia & Lymphoma | England | 320 | 11.43 | 1.512 |
| 15 | Leukemia Research | England | 286 | 26.00 | 2.214 |
| 16 | Journal of Clinical Oncology | USA | 285 | 19.00 | 32.956 |
| 17 | Blood Cancer Journal | England | 211 | 26.38 | 8.023 |
| 18 | Cells | Switzerland | 180 | 11.25 | 4.366 |
| 19 | Cytotherapy | Norway | 169 | 24.14 | 4.218 |
| 20 | Blood Advances | USA | 155 | 22.14 | 4.580 |
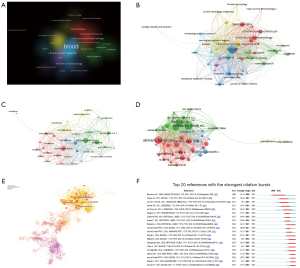
VOSviewer was used to analyze the citation network among these 286 journals. The minimum number of publications was set to 5 for each journal, and then a graphical view was generated. The clusters in diverse colors represent publications concerning diverse research fields (Figure 3A). A dot on the graphical view represents a periodical view, and the line between them represents the cocitation (Figure 3B).
Landmark authors and publications
These 1,030 articles were completed and published by 5,462 authors, and on average, 5.3 researchers worked as a group to publish a manuscript. Van De Donk N, Mutis T and Richardson PG were the three highest-ranked researchers, publishing more than 30 manuscripts.
The software VOSviewer and CiteSpace were used to discover the cooperation and citation relationships among researchers. The researchers who possessed the papers cited more than 450 times (global citation score >450) were known as “Key researchers”. In the graphical view, the larger the dot, the higher the number of citations. The lines between two dots prove the degree of cooperation (Figure 3C,3D). The cited number of publications indicates the contribution of the author and his or her research status in the field. With more than 2000 citations in total, Lokhorst HM, Mutis T and Ahmadi T were the landmark authors, and their contributions in this field were considerable (Figure 3D).
A total of 1,030 publications on CD38-targeting antibody therapy in MM also cited one another over time. The CiteSpace system was applied to analyze 11,572 references, generating a cocitation network of articles (Figure 3E) and the top 20 strongest citation bursts (Figure 3F). The number of citations of these 20 manuscripts increased rapidly during a certain time when they were read, accepted and disseminated diffusely. These papers occupied a key position in this research field.
Visualization of Medical Subject Heading (MeSH) terms and keywords
MeSH terms and keywords help to detect hot issues in the field of research and trace the variation in research focus. VOSviewer was used to analyze and visualize the keywords in the publications (Figure 4A,4B). By using CiteSpace in this course, we fetched the cocitation network, which was separated into 10 clusters (Figure 4C): cluster 0 “flow cytometry”, cluster 1 “daratumumab”, cluster 2 “B cell maturation antigen (BCMA)”, cluster 3 “cell line”, cluster 4 “antitumor activity”, cluster 5 “gene”, cluster 6 “non-Hodegkin lymphoma”, cluster 7 “peripheral blood”, cluster 8 “survival” and cluster 9 “anti-CD38”.
Timeline view and time zone view of CD38 in MM co-citation network
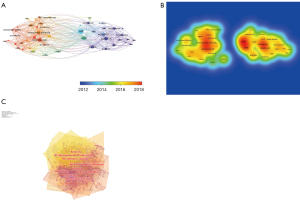
The timeline and timezone views of CD38-targeting antibody therapy in the MM cocitation network were determined with CiteSpace. At the top of the figure, we can see the publication time, and on the right of figure, we can view the terms or keywords in the publications, while nodes on the left transverse lines indicate the behavior of hotspots, and links between them indicate citing. The emergence, popularity and decline of hotspots can be seen.
Furthermore, the clusters in the timeline view disclosed the course of MM development. The evolution of cluster 0 (bone marrow) arose first, showing the usage of CD38-targeting antibodies to treat MM started with the discovery that scientists found CD38-positive myeloma cells to be adhesive to bone marrow. Cluster 1 (monoclonal antibodies), cluster 2 (refractory multiple myeloma), cluster 9 (monoclonal antibody therapy) and cluster 10 (lymphocytic leukemia) were immediate fields of research focus (Figure 5A). The keyword timeline view extracted keywords such as DARA, flow cytometry, cell line and BCMA (Figure 5B).
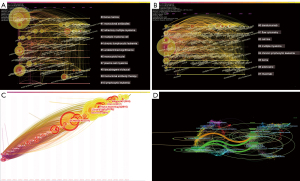
The timezone view also revealed the cocitation network over time demonstrating the evolution of the research field (Figure 5C). Currently, researchers focus on how to choose the proper therapeutic regime to treat RRMM, while CD38-targeting antibodies are becoming increasingly popular and approachable in real-world therapeutic regimens to obtain a better treatment response. The more articles there were in the timezone view, the more important the period was.
The dual-map overlay is a citation overlay that sets left side as citing outline, right side as a cited outline and the link between them as the citation relationship (Figure 5D). This overlay is a work of art that connects different research fields together to enhance our understanding of different specialties.
Discussion
As vividly shown above, bibliometric analysis can provide the visual results that help scientific research personnel who are new to the field improve their command of it. These user-friendly and freely accessible bibliometric software programs can not only uncover milestones in the process and present hotspots but can also courses of disease development. In this study, we presented a bibliometric analysis concerning CD38-targeting antibody therapy in MM.
According to the results obtained above, the numbers of publications on CD38 and MM have increased year by year and increased dramatically after 2015 (Figure 2A); 2015 is a specific point in time that divided the history of research into two parts. Before 2015, the expression of CD38 acted as an indicator of the diagnosis and prognosis of MM and was used to detect the minimal residual disease (MRD) of MM. With the emergence of CD38-targeting antibodies, in vitro and in vivo experiments were conducted to prove the efficiency and safety of this immunotherapy. At the end of 2015, the Food and Drug Administration (FDA) approved DARA (Darzalex, Johnson & Johnson), the world’s first CD38 monoclonal antibody for the treatment of MM (18). Clinical trials were performed to identify a better therapeutic regimen to improve the overall response rate (ORR) of newly diagnosed MM patients and extend progression-free survival (PFS) of RRMM patients. Among all 57 countries/regions, the USA published 450 articles, accounting for the highest proportion (43.7%). It was followed by some European countries, such as Italy, Germany, France and the Netherlands, which processed more than 100 publications each (Figure 2B). Among the top 15 productive institutions, 73.3% are located in the USA (Figure 2G). The USA ranks far ahead of other countries regarding the total number of cited publications, while Denmark has the highest average number of citations (Figure 2F). This result means that the USA is a leading country in research of CD38-targeting antibody therapy in MM, while some European countries, such as Denmark, have published profound and commonly accepted articles (8,12).
In regard to the related journals, VOSviewer was used to analyze 47 of the most-cited journals and divided them into 6 clusters (Figure 3A,3B). One color represents the congeneric cluster referring to the same research field. CiteSpace software was also used to group research fields into 10 clusters (Figure 4C): cluster 1 “flow cytometry”, cluster 2 “daratumumab”, cluster 3 “BCMA”, cluster 4 “cell line”, cluster 5 “antitumor activity”, cluster 6 “gene”, cluster 7 “non-Hodgkin lymphoma”, cluster 8 “peripheral blood”, cluster 9 “survival”, cluster 10 “anti-CD38”. These are 10 major research orientations. MM is the second most common hematological malignancy in Europe, and the number of patients is growing year by year (19). Monoclonal antibodies plus IMiDs and PIs, followed by long-term chemotherapeutic drugs and/or hematopoietic stem cell transplantation (HSCT) and chimeric antigen receptor T cell (CAR-T) therapy, are currently the widely accepted induction treatment (20,21). The anti-CD38 monoclonal antibody showed a good safety profile and favorable efficacy (8,22-24). As the influence of the immune-related microenvironment needs to be confirmed in further clinical practice, anti-CD38 monoclonal antibodies still have research valve. Moreover, the cocitation analysis of CiteSpace sifted some landmark articles (Figure 3E,3F). The works of de Weers M, Lokhorst HM, Dimopoulos MA, Palumbo A, Lonial S were contained in the top 5 in centrality and among the top 20 with citation bursts, indicating the improvement of the response rate in patients with newly diagnosed MM or RRMM with the usage of anti-CD38 monoclonal antibody attracting the attention of researchers (8,11,22-24). For patients in different stages of MM, researchers have been devoted to determining proper therapeutic regimens for first-line or palliative treatment to achieve the best effect with the fewest side-effect. The work of de Weers M and van de Donk NWCJ revealed the mechanism of antitumor activity, suggesting a rule for anti-CD38 monoclonal antibodies in treatment of other hematological malignancies (11,25). Multiple mechanisms contribute to antitumor activity against CD38-positive lymphoma cells, such as ADCC and ADCP. Further clinical studies are required to explore the use of CD38-targeting antibodies to treat non-Hodgkin lymphoma (26). These antibodies were included in cluster 5 “antitumor activity” and cluster 7 “non-Hodgkin lymphoma”. Included in cluster 4 “cell line” and cluster 8 “peripheral blood”, Krejcik et al. found the undiscovered and multi-dimensional immunomodulatory role of DARA (12). Nijhof IS revealed the mechanism and solution of DARA resistance (27). CD38 expression and inherent or drug-induced increased CD55 and CD59 expression influence the outcome of treatment. Suggestions such as choosing a suitable interval time or adding all-trans retinoic acid (ATRA) were given. Further clinical practice is needed to prove their feasibility. Overdijk MB expounded that Fcg receptor-mediated cross-linking induces programmed cell death, which is conductive to the antitumor activity of CD38-targeting antibodies (28). Casneuf T illuminated the influence of NK cells on the safety and efficacy of DARA by performing in vitro and in vivo experiments (29). Although NK cells play an important role in ADCC, their reduction during the treatment with DARA dose not interfere with clinical outcomes. This series of articles helps new researchers in the field to have an overall grasp of the mechanism and importance of CD38-targeting antibodies in treating MM. Bibliometric analysis by CiteSpace revealed that the current hotspots of this field are monoclonal antibodies, refractory MM, idecabtagene vicleucel and BCMA (Figure 5A-5C). BCMA, a transmembrane glycoprotein selectively expressed on mature B cell, accelerate the proliferation, differentiation, maturation and survival of B cells (30). The fact that MM cells markedly express higher BCMA than normal cells provides a new prospect for antibody-based immunotherapy. Idecabtagene vicleucel, an anti-BCMA CAR-T therapy approved by the FDA for four or more prior lines for RRMM therapy, suggests that a promising kind of immunotherapy has matured (31). Researchers are devoting themselves to developing therapeutic regimens to obtain deep and durable responses in refractory MM patients. Efforts have been made to achieve long-term remission and improve the response rate and quality of life of these patients. The dual-map overlay analysis showed that the prime domains of CD38-targeting antibody therapy in MM are medicine and biology (Figure 5D).
In brief, there some potential problems to be addressed in relation to CD38-targeting antibody therapy in MM. The first is to improve the prognosis of CD38-targrting antibody-refractory patients. Novel immunotherapies targeting BCMA, such as CAR-T therapy, antibody-drug conjugates and bispecific T cell engagers, are emerging and will be worth the wait (32-34). The second is to explore other combination regimens containing CD38-targeting antibodies for follow-up treatment (35,36). Rechallenge patients with IMiDs and/or PIs after DARA therapy may be efficacious to those who were previously refractory to IMiDs and/or PIs. This hypothesis remains to be verified in further clinical practice.
Nevertheless, there are some limitations to this research. On the one hand, as we used the data only from the WoSCC, the results of this study might be slightly different if more data are included. On the other hand, the analysis of the development tendency was qualitative and accordingly subjective.
Conclusions
In the past, efforts were applied to elucidate the mechanism and effectiveness of CD38-targeting antibodies in treating MM. Future research hotspots will focus on anti-BCMA CAR-T immunotherapy for patients with RRMM. According to this article, new researchers can discover its course of development and structural relationships in this field.
Acknowledgments
Funding: This work was supported by Wenzhou Science & Technology Bureau (No. ZY2021013).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1962/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet 2015;385:2197-208. [Crossref] [PubMed]
- Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 2007;7:585-98. [Crossref] [PubMed]
- Lin P, Owens R, Tricot G, et al. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol 2004;121:482-8. [Crossref] [PubMed]
- Malavasi F, Funaro A, Roggero S, et al. Human CD38: a glycoprotein in search of a function. Immunol Today 1994;15:95-7. [Crossref] [PubMed]
- Quarona V, Zaccarello G, Chillemi A, et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom 2013;84:207-17. [Crossref] [PubMed]
- Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016;387:1551-60. [Crossref] [PubMed]
- Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016;128:37-44. [Crossref] [PubMed]
- Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med 2015;373:1207-19. [Crossref] [PubMed]
- Overdijk MB, Verploegen S, Bögels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015;7:311-21. [Crossref] [PubMed]
- Nijhof IS, Groen RW, Lokhorst HM, et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015;29:2039-49. [Crossref] [PubMed]
- de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011;186:1840-8. [Crossref] [PubMed]
- Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016;128:384-94. [Crossref] [PubMed]
- Durie BGM, Kumar SK, Usmani SZ, et al. Daratumumab-lenalidomide-dexamethasone vs standard-of-care regimens: Efficacy in transplant-ineligible untreated myeloma. Am J Hematol 2020;95:1486-94. [Crossref] [PubMed]
- Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020;396:186-97. Erratum in: Lancet 2020;396:466. [Crossref] [PubMed]
- van Eck NJ, Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 2017;111:1053-70. [Crossref] [PubMed]
- Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS One 2019;14:e0223994. [Crossref] [PubMed]
- Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A 2004;101:5303-10. [Crossref] [PubMed]
- Poh A. Multiple Myeloma Gets Three New Drugs. Cancer Discov 2016;6:4. [Crossref] [PubMed]
- Dimopoulos MA, Terpos E. Multiple myeloma. Ann Oncol 2010;21:vii143-50. [Crossref] [PubMed]
- Dimopoulos MA, Jakubowiak AJ, McCarthy PL, et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J 2020;10:17. [Crossref] [PubMed]
- Cohen AD, Garfall AL, Stadtmauer EA, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest 2019;129:2210-21. [Crossref] [PubMed]
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016;375:754-66. [Crossref] [PubMed]
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016;375:1319-31. [Crossref] [PubMed]
- Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017;130:974-81. [Crossref] [PubMed]
- van de Donk NW, Janmaat ML, Mutis T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev 2016;270:95-112. [Crossref] [PubMed]
- Vidal-Crespo A, Matas-Céspedes A, Rodriguez V, et al. Daratumumab displays in vitro and in vivo anti-tumor activity in models of B-cell non-Hodgkin lymphoma and improves responses to standard chemo-immunotherapy regimens. Haematologica 2020;105:1032-41. [Crossref] [PubMed]
- Nijhof IS, Casneuf T, van Velzen J, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016;128:959-70. [Crossref] [PubMed]
- Overdijk MB, Jansen JH, Nederend M, et al. The Therapeutic CD38 Monoclonal Antibody Daratumumab Induces Programmed Cell Death via Fcγ Receptor-Mediated Cross-Linking. J Immunol 2016;197:807-13. [Crossref] [PubMed]
- Casneuf T, Xu XS, Adams HC 3rd, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv 2017;1:2105-14. [Crossref] [PubMed]
- Cho SF, Anderson KC, Tai YT, Targeting B. Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front Immunol 2018;9:1821. [Crossref] [PubMed]
- Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med 2021;384:705-16. [Crossref] [PubMed]
- Zhao WH, Liu J, Wang BY, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol 2018;11:141. [Crossref] [PubMed]
- Trudel S, Lendvai N, Popat R, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol 2018;19:1641-53. [Crossref] [PubMed]
- Hipp S, Tai YT, Blanset D, et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 2017;31:1743-51. [Crossref] [PubMed]
- Gavriatopoulou M, Kastritis E, Ntanasis-Stathopoulos I, et al. The addition of IMiDs for patients with daratumumab-refractory multiple myeloma can overcome refractoriness to both agents. Blood 2018;131:464-7. [Crossref] [PubMed]
- Oostvogels R, Jak M, Raymakers R, et al. Efficacy of retreatment with immunomodulatory drugs and proteasome inhibitors following daratumumab monotherapy in relapsed and refractory multiple myeloma patients. Br J Haematol 2018;183:60-7. [Crossref] [PubMed]


