
Preoperative MRI features predict failed breast-conserving surgery: construction of a predictive model
Introduction
Breast cancer is a malignant tumor with the highest and increasing incidence in women in China every year (1), which severely threatens women’s health. Traditional surgery includes complete mastectomy and axillary lymph node dissection, resulting in the destruction of female sexual organs, paresthesia, and upper limb edema and dysfunction in some patients (2). With the improvement of the treatment technique, the need for breast cancer operation is reduced selectively. Thus, breast cancer narrowly escapes the scope of surgery, such that a large number of patients do not have to undergo mastectomy.
Breast-conserving surgery (BCS) is the preferred method for early breast cancer. Several studies have shown that women with breast cancer undergoing breast-conserving therapy (mastectomy combined with adjuvant radiotherapy) have survival rates similar to mastectomy (3-5). Compared to the 50% breast-conserving rate in European and American countries, the proportion of BCS in China for operable breast cancer still has a gap, ranging from 10% to 20% (6-10). However, with the continuous increase in the diagnosis rate of early breast cancer and the change in women’s awareness in China, BCS has become the leading surgical method, and a large number of patients will be protected from unnecessary whole breast removal.
The aim of BCS is the complete removal of the tumor while maintaining a satisfactory breast shape. Presently, doctors subjectively judge whether BCS is feasible mainly according to the results of ultrasound, magnetic resonance imaging (MRI), and mammography. Previous studies have shown that within 12 months after BCS, radical mastectomy was performed in 3–17% of cases, and secondary BCS was performed in 11–18% of cases (8,11,12). In the secondary resection, tumor remnants were detected in 20% of breast-conserving cases and 59% of radical resection cases, while in secondary breast-conserving resection, the ipsilateral recurrence rate was almost three times that of breast-conserving cases, regardless of the presence of tumor residues in the second surgery (11). This phenomenon indicated that the accuracy of doctors’ subjective judgment on the feasibility of BCS is not high. Also, due to the lack of objective quantitative standards and the high reliance on doctors’ experience, the secondary surgery rate after BCS varied across hospitals (8). When lumpectomy is performed, if the breast-conserving operation fails, the surgical plan should be changed to complete mastectomy. This increases medical costs, delays adjuvant treatment, and has poor cosmetic results. Therefore, accurate preoperative prediction of the feasibility of BCS helps to formulate an appropriate surgical plan and reduce the will violation of the patient. To evaluate the feasibility of BCS, it is essential to establish objective and quantitative criteria for clinical practice. Currently, no relevant standards have been reported worldwide.
In previous studies, the maximum diameter of the tumor is the most commonly used indicator for tumor size (13). However, due to the irregular shape, the maximum diameter often fails to reflect is the actual size of the tumor. Some studies have shown that the measurement of breast tumor diameter alone cannot improve the success rate of BCS and reduce ipsilateral recurrence or the rate of secondary BCS (12). Interestingly, MRI is significantly more accurate than ultrasound and mammography in measuring the tumor size and range (12,14-17), and the measurement of breast tumor volume before and after neoadjuvant therapy is reliable (18,19). It has been speculated that the main factors influencing the feasibility of BCS are tumor size, location, proportion in the breast, and patient willingness. Therefore, measuring and screening effective imaging indicators to predict failed BCS preoperatively is a major concern. Previously, only MRI signs were used to predict the positive margin to judge the success of BCS surgery (20), but no studies have reported the combination of imaging modalities with multiple factors such as tumor volume and location for accurate prediction.
Therefore, the present study proposed to explore the preoperative MRI features associated with failed BCS and construct an MRI-based predictive model to help the clinicians determine the approach for BCS. We present the following article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1919/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the institutional review board of Peking University Cancer Hospital (China) (ID. 2017KY96), and patient informed consent was waived due to its retrospective nature.
We included pathologically proven breast cancer patients in our hospital who planned to retain their breasts and receive preoperative magnetic resonance (MR) examination. The follow-up was required for at least 2 years. Patients with incomplete clinical or follow-up data or with unqualified or missing MRI were excluded. Both groups of patients who underwent surgery directly and received neoadjuvant therapies were included. Subsequently, the preoperative MRI after neoadjuvant therapies were used.
A total of 295 women between March 2015 and July 2016 were included: 265 underwent successful BCS, and 30 presented failed BCS. Failure to retain breast is defined as meeting any of the following three criteria: (I) positive margins identified by intraoperative pathology of frozen sections; (II) ipsilateral breast recurrence within 2 years after BCS; (III) the appearance evaluation of the breast surgery is not ideal (poor and very poor).
Failed and successful BCS patients were at a ratio of 1:3 matched according to age, neoadjuvant therapy, and hormone receptor expression. Finally, a total of 120 preplanned BCS patients (age: 45.7±10.3 years; range, 22–75 years) were included in the analysis.
Patients were chronologically divided into training groups (including 15 patients with failed BCS and 45 patients with successful BCS) for model construction and testing group (including 15 patients with failed BCS and 45 patients with successful BCS) for model validation.
MR protocol
MR examination was carried out on a 1.5T MRI scanner within 2 weeks before surgery. Four-channel phased-array breast coil (Echospeed plus and excite II, GE Medical Systems, Milwaukee, WI, USA) was used. The examination program involved dynamic enhanced sagittal, three-dimensional vibrant SPGR sequence (TR =6.4 ms, TE =3.0 ms, TI =7.0 ms, flip angle =10°, slice thickness =4 mm, no interlayer gap, matrix size =256×256, field of vision =20–22 cm, NEX =1, ZIP2, scan time per acquisition =68 s), and an axial, fat suppression, T1 weighted pulse sequence enhancement. The vibration sequence was repeated six times successively, and the dynamic acquisition was carried out in the first phase before contrast enhancement and five phases after contrast enhancement. The contrast agent (Gd-DTPA) was injected into the anterior elbow vein by a power syringe at a speed of 2.0 mL/s [based on the patient’s weight (0.2 mmol/kg)], and flushed with saline. First, the T1 weighted pre-contrast scan with fat saturation was collected initially. Then, 2 min after injection of contrast medium, the first contrast medium was collected and scanned. Subsequently, four postcontrast images were obtained every 90 s, and five post-contrast images were obtained (t=2, 3.5, 5, 6.5, and 8 min).
MRI evaluation
All MR images were examined by two independent radiologists (YH Qu with 10-year experience and RJ Sun with 5-year experience of breast MR diagnosis) using 3D slicer software (version 4.8.1).
Both radiologists were blinded to the clinical data and follow-up information of enrolled patients. The region of interest (ROI) was marked manually using 3D slicer software, delineating the tumor boundary layer by layer, and the tumor volume was calculated. The ROI is delineated along tumor edges, including surrounding burrs and bands, in the first phase of the postcontrast T1-weighted imaging contrast enhancement sequence (21). In patients after neoadjuvant therapy, if the first phase of postcontrast T1 dynamic enhancement (early dynamic enhancement) shows tumor signals, then the principles are the same as before the neoadjuvant therapy. If no clear tumor signal was found in the first phase of postcontrast T1 dynamic enhancement after neoadjuvant therapy, the disease was characterized by MR-pathological complete response (MR-pcr), and the tumor volume was 0. The 3D slicer software was used to measure the volume of the affected breast, and the threshold method was used to segment the affected breast and automatically calculate the volume of the breast. The safety of BCS in multifocal breast lesions has been demonstrated previously (21), which prompted us to assess whether the lesions are multifocal. Multifocal lesions are defined as those located within the same quadrant and multicentric tumors residing in different quadrants. The preoperative MRI features were assessed on the site of the tumor (upper-outer quadrant, upper-inner quadrant, lower-outer quadrant, and lower-inner quadrant), the lesion type (mass and non-mass enhancement), the existence of multifocality, and the volume features (the volume of lesion, the volume of affected breast, and the ratio of the two) (Figure 1).
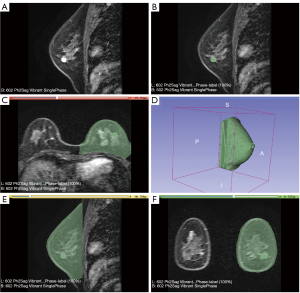
The agreement between the two radiologists was evaluated. The average of measurement was used for continuous variables for subsequent analysis. For categorical variables, a third experienced radiologist was introduced for arbitration.
Evaluation of breast appearance
The surgeons evaluated the appearance of the breast 2 years after BCS. Excellent appearance was defined when the treated breast was almost identical to the untreated; good appearance was defined when the treated breast was slightly different from the untreated breast; poor appearance was defined as the obvious difference between the two sides without major distortion; very poor appearance was defined as severely distorted treated breast. The breast appearance was evaluated by two surgeons, and a third surgeon was introduced for arbitration in the case of divergence.
Statistical analysis
The differences in MRI features between failure and successful BCS groups were compared. Continuous variables were compared using an independent t-test or Mann-Whitney U test. Categorical variables were compared using chi-square test or Fisher’s exact test. The statistically significant factors were substituted into the multivariate logistic model to select independent factors to predict failed BCS. Univariate analysis was conducted using the whole sample to explore the potentially useful factors associated with failed BCS, followed by multivariate analysis using the training group to construct an MR-based model to predict failed BCS; this model was validated using the testing group. Receiver operating characteristic (ROC) curve was drawn, and the area under the curve (AUC) was calculated to evaluate the diagnostic capability of the MR-based model in predicting failed BCS. The cutoff was determined by the maximum Youden’s method; then, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and total accuracy were calculated for failed BCS. The intra-class correlation coefficient (ICC) was calculated to evaluate the inter-observer agreement: 0.0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00 indicated no, poor, moderate, substantial, and perfect agreement, respectively. SPSS 22.0 was used for statistical analysis, and a two-sided P<0.05 indicated statistical significance.
Results
Patient characteristics
The cohort comprised 30 patients with failed BCS and 90 successful BCS. The median follow-up after surgery was 43 months. Patient demographics and tumor characteristics are listed in Table 1. The reasons for failed BCS in 30 patients were due to the positive margins based on the intraoperative rapid pathological diagnosis.
Table 1
| Variables | Values |
|---|---|
| Age (years) | 45.7±9.3 |
| HR(+)* | 96 (80.0%) |
| HER2(+)** | 20 (16.7%) |
| BCS | |
| Successful | 90 (75.0%) |
| Failure | 30 (25%) |
| Neoadjuvant therapies | |
| No | 44 (36.7%) |
| Yes | 76 (63.3%) |
| Lesion type | |
| Mass | 106 (88.3%) |
| Non-mass | 14 (11.7%) |
| Tumor location | |
| Upper-outer quadrant | 63 (52.5%) |
| Upper-inner quadrant | 21 (17.5%) |
| Lower-outer quadrant | 27 (22.5%) |
| Lower-inner quadrant | 9 (7.5%) |
| Volume features | |
| Volume of lesion (mm3) | 2,081.50±3,221.16 |
| Volume of affected breast (mm3) | 794,398.53±322,544.63 |
| Ratio of lesion and breast volume (×10−4)*** | 29.03±50.23 |
| Multifocality | |
| No | 98 (81.7%) |
| Yes | 22 (11.3%) |
Data are represented as mean ± SD or n (%). *, HR: hormone receptor, including ER and PR, was determined on the biopsy specimens or surgically excised specimens. ER and PR were evaluated by the percentages of stained tumors. The positivity for ER or PR was defined as ≥10% stained tumor cells, and either ER- or PR-positive was regarded as HR-positive. **, HER2: human epidermal growth factor receptor type 2, determined with respect to biopsy specimens or surgically excised specimens. HER2 immunohistochemistry was scored using the ASCO/CAP criteria to assess the intensity and completeness of membrane staining. A score of 0/+ was considered negative, and 3+ was considered positive. A score of 2+ was further evaluated with FISH to determine the HER2 status. If the ratio of the HER2 gene signal to the chromosome 17 probe signal was >2.2, the tumor was classified as HER2 positive. ***, ratio of lesion and breast volume: the ratio of lesion and breast volume, calculated as Vlesion/Vbreast. ER, estrogen receptor; PR, progesterone receptor; ASCO/CAP, American Society of Clinical Oncology and the College of American Pathologists; FISH, fluorescence in situ hybridization; BCS, breast-conserving surgery.
The distribution of age, neoadjuvant therapy, hormone receptor expression, and HER2 expression was similar between the successful and failure groups (all P>0.05; Table 2).
Table 2
| Variable | Successful group (n=90) | Failure group (n=30) | P |
|---|---|---|---|
| Age (years) | 46.2±8.8 | 44.3±10.6 | 0.684 |
| Neoadjuvant therapies | 1.000 | ||
| No | 33 (36.7%) | 11 (36.7%) | |
| Yes | 57 (63.3%) | 19 (63.3%) | |
| HR* | 0.572 | ||
| Positive | 16 (30%) | 4 (13.3%) | |
| Negative | 74 (70%) | 26 (86.7%) | |
| HER2** | 1.000 | ||
| Positive | 18 (20%) | 6 (20%) | |
| Negative | 72 (80%) | 24 (80%) | |
| Lesion type | 0.007 | ||
| Mass | 84 (93.3%) | 22 (73.3%) | |
| Non-mass | 6 (6.7%) | 8 (26.7%) | |
| Tumor location | 0.416 | ||
| Upper-outer quadrant | 50 (55.6%) | 13 (43.3%) | |
| Upper-inner quadrant | 13 (14.4%) | 8 (26.7%) | |
| Lower-outer quadrant | 21 (23.3%) | 6 (20.0%) | |
| Lower-inner quadrant | 6 (6.7%) | 3 (10.0%) | |
| Volume features | |||
| Volume of lesion (mm3) | 1,088.36±1,636.37 | 5,060.92±4,702.65 | <0.001 |
| Volume of affected breast (mm3) | 787,922.16±323,872.50 | 813,827.65±323,214.31 | 0.598 |
| Ratio of lesion and breast volume (×10−4)*** | 15.66±23.48 | 71.05±79.82 | <0.001 |
| Multifocality | <0.001 | ||
| No | 83 (92.2%) | 15 (50.0%) | |
| Yes | 7 (7.8%) | 15 (50.0%) | |
| Pathological type | 0.721 | ||
| Invasive ductal carcinoma | 80 (89.0%) | 25 (83.3%) | |
| Ductal carcinoma in situ | 2 (2.2%) | 1 (3.3%) | |
| Invasive lobular carcinoma | 6 (6.7%) | 2 (6.7%) | |
| Others | 2 (2.2%) | 2 (6.7%) |
Data are represented as mean ± SD or n (%). *, HR: hormone receptor, including ER and PR, was determined on the biopsy specimens or surgically excised specimens. ER and PR were evaluated by the percentages of stained tumors. The positivity for ER or PR was defined as ≥10% stained tumor cells, and either ER- or PR-positive was regarded as HR-positive. **, HER2: human epidermal growth factor receptor type 2, determined with respect to biopsy specimens or surgically excised specimens. HER2 immunohistochemistry was scored using the ASCO/CAP criteria to assess the intensity and completeness of membrane staining. A score of 0/+ was considered negative, and 3+ was considered positive. A score of 2+ was further evaluated with FISH to determine the HER2 status. If the ratio of the HER2 gene signal to the chromosome 17 probe signal was >2.2, the tumor was classified as HER2 positive. ***, ratio of lesion and breast volume: the ratio of lesion and breast volume, calculated as Vlesion/Vbreast. ER, estrogen receptor; PR, progesterone receptor; ASCO/CAP, American Society of Clinical Oncology and the College of American Pathologists; FISH, fluorescence in situ hybridization; BCS, breast-conserving surgery; MRI, magnetic resonance imaging.
Comparison of MRI features between successful and failure BCS groups
Significantly more non-mass lesions (26.7% vs. 6.7%, P=0.007) and multifocality (50.0% vs. 7.8%, P<0.001) were observed in the failure BCS group compared to the successful group. Also, the volume of the lesion (5,060.92±4,702.65 vs. 1,088.36±1,636.37 mm3, P<0.001) and the ratio of lesion and breast volume (0.0071±0.0079 vs. 0.0015±0.0023, P<0.001) was significantly larger in the group than the successful group. The data are listed in Table 2.
Construction of MR-based model for predicting failed BCS in the training group and the validation of the model in the testing group
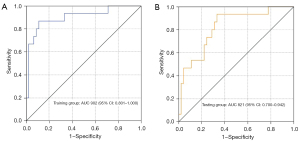
In the training group, the multivariate logistic model showed that the ratio of lesion and breast volume and multifocality of the tumor were independent factors associated with failed BCS [odds ratios (ORs) were 1.044, 95% confidence interval (CI): 1.016–1.074] and 11.161 (95% CI: 1.739–71.652), respectively. An MR-based model was established as Y=0.044 × the ratio of lesion and breast volume (104) + 2.412 × multifocality, yielding an AUC of 0.902 (95% CI: 0.801–1.000) for predicting failed BCS. A cutoff of 2.3 was selected; Y>2.3 indicated failed BCS, while Y≤2.3 indicated successful BCS. The sensitivity, specificity, PPV, NPV, and total accuracy for predicting failed BCS were 53.3%, 88.9%, 61.5%, 85.1%, and 80%, respectively.
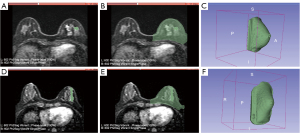
In the testing group, the AUC was 0.821 (95% CI: 0.700–942), and the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and total accuracy for predicting failed BCS was 46.7%, 88.9%, 58.3%, 83.3%, and 78.3%, respectively. Two cases with successful and failed BCS were showed in Figure 2.
The diagnostic performance of the constructed model for predicting failed BCS in the training and testing groups is summarized in Table 3 and Figure 3.
Table 3
| Group | AUC (95% CI) | Cutoff | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| Training | 0.902 (0.801–1.000) | 2.3 | 53.3 (8/15) | 88.9 (40/45) | 61.5(8/13) | 85.1 (40/47) | 80 (48/60) |
| Testing | 0.821 (0.701–0.942) | – | 46.7 (7/15) | 88.9 (40/45) | 58.3(7/12) | 83.3 (40/48) | 78.3 (47/60) |
BCS, breast-conserving surgery; AUC, area under the receiver operating characteristic curve; PPV, positive predictive value; NPV, negative predictive value.
Inter-observer agreement
A perfect interobserver agreement was observed for the volume of the lesion (ICC =0.874), the volume of the breast (ICC =0.828), the lesion type (ICC =0.959), the multifocality (ICC =0.860), and the site of the tumor (ICC =0.824). A substantial agreement was observed for the ratio of the lesion and breast volume (ICC =0.787).
Discussion
The present study demonstrated that preoperative MRI parameters of the ratio of lesion and breast volume and multifocality were associated with failed BCS. The study also constructed a multivariate MR-based model for predicting failed BCS and tested the model using samples from the same center.
Currently, when clinicians decide to conduct BCS, the size and location of the tumor, combined with the patient’s will, are considered. Importantly, the doctors demonstrated high accuracy of the feasibility of breast conservation preoperatively, and their decision directly affects whether the patient can receive the most suitable treatment and the patient’s subjective willingness to choose the type of operation. According to the data of this study, the failure rate of BCS is about 10%. The constructed combination model selects 73.3% of patients who received but may not be suitable for BCS, deeming it to be of clinical significance.
The objective indicators that judge the feasibility of breast conservation can overcome the influence of doctors’ subjective experience. The primary method for measuring the tumor volume is to combine doctor delineation and software to calculate the volume automatically; the finding was consistent among the measurers (18,22,23). A few studies have reported the measurement of breast volume by MRI; nonetheless, these comprised a small sample. The principle is to scan the area of each layer of the breast tissue at specific intervals of height and summarize the data (24). Because MRI measurement of breast tumor and volume highly depends on the judgment of the researchers, especially the image measurement after new adjuvant therapy, few studies are based on this parameter in the evaluation of breast tumor surgery type. The measurement method of this study is simple and easy, and unaffected by the machine and scanning parameters. Perfect interobserver agreement was observed to measure tumor volume, breast volume, and the ratio of the two parameters.
Nevertheless, the present study has some limitations. First, it was a single-center, retrospective design, consisting of both patients with and without neoadjuvant therapies. Due to the relatively small samples that failed breast conservation, we used a 1:3 matched ratio to control bias and improve the statistical power of the study. Second, the model constructed in this study needs to be validated in external independent samples. In addition, whether the clinicians can improve the accuracy of preoperative judgment for BCS using the model is a major concern.
Conclusions
This study established an MRI-based preoperative breast-conserving feasibility prediction model using simple and easy-to-measure parameters. After further verification in more samples, the predictive model with high diagnostic accuracy may provide an effective objective reference for clinicians to predict the feasibility of breast conservation.
Acknowledgments
Funding: This study was supported by the Beijing Municipal Administration of Hospitals Incubating Program (No. PX2018041), Beijing Municipal Science and Technology Commission (No. Z181100001918001), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201803), Beijing million Talents Project (No. 2017A13), and Beijing Hospitals Authority’ Ascent Plan (No. 20191103).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1919/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1919/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1919/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1919/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the institutional review board of Peking University Cancer Hospital (China) (ID. 2017KY96), and patient informed consent was waived due to its retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Lacour J, Le M, Caceres E, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer 1983;51:1941-3. [Crossref] [PubMed]
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-50. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg 2015;262:434-9. [Crossref] [PubMed]
- Chiba A, Hoskin TL, Heins CN, et al. Trends in Neoadjuvant Endocrine Therapy Use and Impact on Rates of Breast Conservation in Hormone Receptor-Positive Breast Cancer: A National Cancer Data Base Study. Ann Surg Oncol 2017;24:418-24. [Crossref] [PubMed]
- Spilsbury K, Semmens JB, Saunders CM, et al. Subsequent surgery after initial breast conserving surgery: a population based study. ANZ J Surg 2005;75:260-4. [Crossref] [PubMed]
- Porter G, Wagar B, Bryant H, et al. Rates of breast cancer surgery in Canada from 2007/08 to 2009/10: retrospective cohort study. CMAJ Open 2014;2:E102-8. [Crossref] [PubMed]
- Zhang BN, Zhang B, Tang ZH, et al. 10-year changes and development of surgical treatment for breast cancer in China. Chinese Journal of Oncology 2012;34:582-7. [PubMed]
- Bodilsen A, Bjerre K, Offersen BV, et al. The Influence of Repeat Surgery and Residual Disease on Recurrence After Breast-Conserving Surgery: A Danish Breast Cancer Cooperative Group Study. Ann Surg Oncol 2015;22:S476-85. [Crossref] [PubMed]
- Shin HC, Han W, Moon HG, et al. Limited value and utility of breast MRI in patients undergoing breast-conserving cancer surgery. Ann Surg Oncol 2012;19:2572-9. [Crossref] [PubMed]
- Youn I, Choi S, Choi YJ, et al. Contrast enhanced digital mammography versus magnetic resonance imaging for accurate measurement of the size of breast cancer. Br J Radiol 2019;92:20180929. [Crossref] [PubMed]
- Bhattacharyya M, Ryan D, Carpenter R, et al. Use of magnetic resonance imaging to plan breast conserving surgery following neoadjuvant chemotherapy for early breast cancer. J Clin Oncol 2004;22:S24. [Crossref]
- Uematsu T, Yuen S, Kasami M, et al. Comparison of magnetic resonance imaging, multidetector row computed tomography, ultrasonography, and mammography for tumor extension of breast cancer. Breast Cancer Res Treat 2008;112:461-74. [Crossref] [PubMed]
- Boetes C, Mus RD, Holland R, et al. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology 1995;197:743-7. [Crossref] [PubMed]
- Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 1999;213:881-8. [Crossref] [PubMed]
- Lorenzon M, Zuiani C, Londero V, et al. Assessment of breast cancer response to neoadjuvant chemotherapy: is volumetric MRI a reliable tool? Eur J Radiol 2009;71:82-8. [Crossref] [PubMed]
- Zhang A, Li J, Wang W, et al. A comparison study between gross tumor volumes defined by preoperative magnetic resonance imaging, postoperative specimens, and tumor bed for radiotherapy after breast-conserving surgery. Medicine (Baltimore) 2017;96:e5839. [Crossref] [PubMed]
- Bahl M, Baker JA, Kinsey EN, et al. MRI predictors of tumor-positive margins after breast-conserving surgery. Clin Imaging 2019;57:45-9. [Crossref] [PubMed]
- Oh JL, Dryden MJ, Woodward WA, et al. Locoregional control of clinically diagnosed multifocal or multicentric breast cancer after neoadjuvant chemotherapy and locoregional therapy. J Clin Oncol 2006;24:4971-5. [Crossref] [PubMed]
- den Hartogh MD, Philippens ME, van Dam IE, et al. MRI and CT imaging for preoperative target volume delineation in breast-conserving therapy. Radiat Oncol 2014;9:63. [Crossref] [PubMed]
- Jacobs MA, Stearns V, Wolff AC, et al. Multiparametric magnetic resonance imaging, spectroscopy and multinuclear (23Na) imaging monitoring of preoperative chemotherapy for locally advanced breast cancer. Acad Radiol 2010;17:1477-85. [Crossref] [PubMed]
- Hussain Z, Roberts N, Whitehouse GH, et al. Estimation of breast volume and its variation during the menstrual cycle using MRI and stereology. Br J Radiol 1999;72:236-45. [Crossref] [PubMed]


