
A retrospective study based on SEER database: not all high-risk factors are equal for stage II colon cancer
Introduction
Colon cancer has become an increasingly common malignant tumor in human. Annual global incidence is approximately 1.4 million with nearly 700,000 deaths (1,2). At the time of initial diagnosis, stage II colon cancer counts for approximately 25% of all cases. Surgical resection is the mainstay of treatment for stage II colon cancer. However, whether or not to give adjuvant chemotherapy is still a controversial issue, although the benefits of fluorouracil (FU)-based adjuvant chemotherapy in reducing recurrence and prolonging survival have been well established in stage III colon cancers (3). A Cancer Care Ontario Systematic Review reported that adjuvant chemotherapy have an overall survival (OS) benefit for patients with completely resected stage III colon cancer, but a clear OS benefit has not been shown for stage II colon cancer (4). Similarly, a randomized controlled trial performed by André et al. (3) have indicated that the OS of patients with stage II colon cancer did not benefit from adjuvant chemotherapy [HR =0.84; 95% confidence interval (CI): 0.62–1.14; P=0.258]. And another trial has also supported that adjuvant chemotherapy did not increase the OS of stage II colon cancer (5). However, some retrospective studies and meta-analysis have found that adjuvant chemotherapy can increase the OS of stage II colon cancer with some high-risk factors (HRFs) and reduce the risk of recurrence. And, some HRFs have been reported previously, such as T4 tumor, perforation, lymphatic vascular invasion (LVI), perineural invasion (PNI), less than 12 lymph nodes (LN) examined, high-grade tumors, positive margins, and obstruction. Adjuvant chemotherapy has been recommended for colon cancer patients with HRFs (6,7). Therefore, there is still no sufficient evidence for this controversial issue. In clinical practice, the decision of adjuvant chemotherapy is to some extent related to the doctor’s personal judgment. Meanwhile, the shortcomings of the current studies are still obvious. Novel HRFs have been insufficiently investigated, more factors, such as tumor location, mucin-producing tumors, tumor deposition, should be included in HRFs and taken into comprehensive analysis. Potential correlations among these HRFs have been ignored as well. And, existing studies have failed to quantify the degree of risk conferred by each HRF, and to evaluate the survival benefit of adjuvant chemotherapy based on each HRF and its combinations.
In general, patients with stage II colon cancer have a favorable prognosis with 5-year OS rate above 80%. Most of these patients will never develop recurrences (8). During the long survival period, more factors other than tumor stage were involved in the development of the outcome. Thus, American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) cannot effectively predict the long-term survival rate of patients, underscoring the need to explore prognostic and predictive models to facilitate the identification of patients for additional intervention. Given increasing value of multiple variables, including tumor location, mucin-producing tumors, tumor deposition and marital status (MS), more HRFs have been noticed gradually. But, of note, very few studies have focused on the nomogram implementation. Therefore, our research is dedicated to identify more HRFs, quantify the degree of risk each HRF confers, assess the survival benefits that each HRF and its combinations can obtain from chemotherapy. And based on the identified HRFs, we attempt to establish an effective and stable predictive model. These results will hopefully provide systematic and effective guidance for clinical practice.
The Surveillance, Epidemiology, and End Results (SEER) program, a clinical database funded by the National Cancer Institute (NCI), collects data on cancer incidence and survival from U.S. cancer registries. SEER routinely collects and publishes data on patient-specific and tumor-specific characteristics. Information collected for each case includes patient demographics, primary tumor site, tumor morphology, stage at diagnosis, treatment course, follow-up for vital status, and cause of death. SEER uses the Population Estimates Program data of the United States Census Bureau and U.S. mortality data, collected and maintained by the National Center for Health Statistics, for population counts (9). We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1779/rc).
Methods
Study population
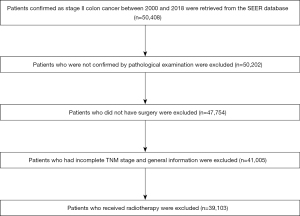
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The clinical variables of patients confirmed as stage II colon cancer between 2000 and 2018 were retrieved from the SEER database, a program stablished by NCI aiming for comprehensively national-level clinical investigation (10). Inclusion criteria were: (I) stage II colon cancer (site recode, international classification of diseases for oncology (ICD-O-3/WHO 2009); (II) diagnosis confirmed by pathological examination; (III) surgery performed in each case. Exclusion criteria were: (I) incomplete information on TNM stage and general information; (II) undergoing radiotherapy and other adjuvant treatments besides chemotherapy (Figure 1).
Chemotherapy and outcome
OS was the primary outcome in this study and was calculated as the time from surgery to death or last date of study follow-up. Data classifying receipt of chemotherapy were limited to yes versus no.
Statistical analyses
Univariate and multivariate Cox regression analysis were performed to identify independent risk factors affecting the OS, and after propensity score adjustment, death risk ratio of each HRF was calculated. Survival analysis was used to evaluate whether each HRF and its combinations could benefit from chemotherapy. Then, the HRFs identified were used to develop a nomogram model. The comparison between the nomogram prediction and observed outcomes was assessed by the concordance index (C-index). The calibration plot was used for visualized comparison between predicted and actual prognosis. Furthermore, the power of nomogram model was compared to AJCC TNM in both receiver operating characteristic curve (ROC) and decision curve analysis (DCA). All analysis was performed by R Studio software 1.4.1106 and IBM SPSS Statistics 24 software. All statistical tests in this study were two-sided, conducted at a significance level of five percent (a =0.05).
Results
Population and HRFs
A total of 39,103 patients with stage II colon cancer, who had received surgery and were diagnosed pathologically during 2000 to 2018, were included in this study. Through univariate and multivariate COX regression analysis, T4 tumors, poorly/undifferentiated tumors, positive carcinoembryonic antigen (CEA), PNI, tumor deposition, LN examination less than 12, advanced age, unmarried status, mucin-producing tumors, and left tumors were identified as the independent risk factors associated with the OS of stage II colon cancer (Table 1). In our study, all independent risk factors were considered as HRFs.
Table 1
| Variables | Total | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Sex | 0.988 | 0.941–1.037 | 0.632 | |||||
| Male | 19,640 | – | – | – | ||||
| Female | 19,463 | – | – | – | ||||
| Age (years) | 1.692 | 1.615–1.773 | <0.001 | |||||
| ≤49 | 3,100 | Reference | ||||||
| 50–64 | 9,765 | 1.223 | 1.078–1.387 | 0.002 | ||||
| ≥65 | 26,238 | 2.317 | 2.065–2.601 | <0.001 | ||||
| Race | 0.949 | 0.914–0.986 | 0.007 | 0.975 | 0.939–1.013 | 0.200 | ||
| White | 31,607 | – | – | – | ||||
| Black | 4,188 | – | – | – | ||||
| Asian | 2,888 | – | – | – | ||||
| American Indian | 301 | – | – | – | ||||
| Unknown | 119 | – | – | – | ||||
| Differentiation grade | 1.141 | 1.103–1.180 | <0.001 | |||||
| Well | 2,846 | Reference | ||||||
| Moderate | 29,138 | 1.123 | 1.017–1.241 | 0.022 | ||||
| Poorly | 5,346 | 1.265 | 1.129–1.418 | <0.001 | ||||
| Undifferentiated | 1,083 | 1.230 | 1.036–1.459 | 0.018 | ||||
| Unknown | 690 | 1.424 | 1.183–1.713 | <0.001 | ||||
| AJCC T | 1.758 | 1.701–1.817 | <0.001 | |||||
| T3 | 33,133 | Reference | ||||||
| T4a | 3,477 | 2.192 | 2.046–2.349 | <0.001 | ||||
| T4b | 2,493 | 2.637 | 2.448–2.840 | <0.001 | ||||
| CEA | 1.139 | 1.321–1.472 | <0.001 | |||||
| Negative | 30,243 | Reference | ||||||
| Positive | 8,860 | 1.287 | 1.218–1.359 | <0.001 | ||||
| PNI | 1.763 | 1.623–1.915 | <0.001 | |||||
| No | 36,737 | Reference | ||||||
| Yes | 2,366 | 1.527 | 1.404–1.661 | <0.001 | ||||
| TD | 2.163 | 1.924–2.433 | <0.001 | |||||
| No | 38,182 | Reference | ||||||
| Yes | 921 | 1.744 | 1.549–1.963 | <0.001 | ||||
| Regional nodes examined | 2.087 | 1.970–2.210 | <0.001 | |||||
| ≥12 | 33,642 | Reference | ||||||
| <12 | 5,461 | 1.925 | 1.816–2.042 | <0.001 | ||||
| MS | 1.535 | 1.461–1.613 | <0.001 | |||||
| Married | 19,711 | Reference | ||||||
| Unmarried | 19,392 | 1.312 | 1.248–1.380 | <0.001 | ||||
| Histology | 1.173 | 1.052–1.307 | <0.001 | |||||
| Others | 38,407 | Reference | ||||||
| Mucin-producing | 399 | 1.533 | 1.254–1.874 | <0.001 | ||||
| Signet ring cell | 297 | 0.979 | 0.745–1.288 | 0.880 | ||||
| Location | 1.124 | 1.096–1.153 | <0.001 | |||||
| Right | 19,267 | Reference | ||||||
| Transverse | 4,358 | 1.033 | 0.951–1.123 | 0.440 | ||||
| Left | 15,478 | 1.343 | 1.273–1.416 | <0.001 | ||||
OS, overall survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; CEA, carcinoembryonic antigen; PNI, perineural invasion; TD, tumor deposits; MS, marital status.
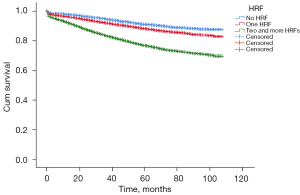
Among all the patients, 5,547 (14.1%) had no HRFs, and 33,556 (85.9%) had at least one HRF, of which 13,066 (33.4%) had only one HRF, 20,490 (52.4%) had two or more HRFs. In our study, 33,339 (85.2%) patients did not receive chemotherapy. The adjusted overall mortality hazard ratios (HR) for each of these HRFs were listed in Table 2. T4b tumors were the highest risk for reduced OS (HR =2.821; 95% CI: 1.949–4.082), the second was mucin-producing tumors (HR =2.412; 95% CI: 1.326–4.388), and the third was LN examined less than 12 (HR =2.200; 95% CI: 1.786–2.710). By the deadline, the OS of patients without any of the HRFs was 91.2%; the OS of patients with at least one HRF was 82%. And a cumulative effect on OS was observed: compared with patients with no HRFs, those with one HRF and two or more HRFs both have a significant decrease in OS (HR =1.381; 95% CI: 1.247–1.529 and HR =2.827; 95% CI: 2.575–3.103, respectively; Figure 2).
Table 2
| HRF | HR | 95% CI | P |
|---|---|---|---|
| T4a tumors | 2.094 | 1.550–2.828 | <0.001 |
| T4b tumors | 2.821 | 1.949–4.082 | <0.001 |
| Positive CEA | 1.317 | 1.087–1.596 | 0.005 |
| PNI | 1.827 | 1.262–2.644 | 0.001 |
| TD | 1.319 | 0.589–2.951 | 0.243 |
| <12 LN examined | 2.200 | 1.786–2.710 | <0.001 |
| Unmarried status | 1.422 | 1.256–1.610 | <0.001 |
| Mucin-producing tumors | 2.412 | 1.326–4.388 | 0.004 |
| Poorly/undifferentiated tumors | 1.125 | 0.910–1.391 | 0.278 |
| Left tumors | 1.183 | 1.027–1.364 | 0.020 |
HR, hazard ratio; HRF, high-risk factor; CI, confidence interval; CEA, carcinoembryonic antigen; PNI, perineural invasion; TD, tumor deposits; LN, lymph nodes.
Chemotherapy and HRFs
Among the patients without any HRF, 465 (8.3%) had received chemotherapy, but they did not gain survival benefits (HR =0.888; 95% CI: 0.643–1.228; P=0.473). Among the patients with one or more HRFs, 5,299 (15.7%) had received adjuvant chemotherapy, and they received significant survival benefit (HR =0.860; 95% CI: 0.802–0.922; P<0.001). Table 3 showed the covariates-adjusted OS HR for each HRF based on the receipt of adjuvant chemotherapy. Not every subgroup of patients with each HRF could benefit from chemotherapy, except for T4 tumors (HR =0.566; 95% CI: 0.433–0.741; P<0.001) and poorly/undifferentiated tumors (HR =0.468; 95% CI: 0.237–0.924; P=0.029). Additionally, for those with more than one HRF, only combinations of HRFs including T4 tumors or poorly/undifferentiated tumors showed a benefit from adjuvant chemotherapy.
Table 3
| HRF | HR (95% CI) | P |
|---|---|---|
| T4 tumors | 0.566 (0.433–0.741) | <0.001 |
| Poorly/undifferentiated tumor | 0.468 (0.237–0.924) | 0.029 |
| Positive CEA | 0.669 (0.371–1.208) | 0.183 |
| Positive PNI | 0.421 (0.101–1.762) | 0.236 |
| TD | 1.146 (0.285–4.602) | 0.848 |
| <12 LN examined | 0.682 (0.376–1.238) | 0.208 |
| Unmarried status | 1.013 (0.745–1.377) | 0.933 |
| Mucin-producing tumors | 0.044 (0.001–689.559) | 0.526 |
| Left tumors | 0.831 (0.614–1.125) | 0.231 |
| T4 tumors + left tumors | 0.527 (0.337–0.826) | 0.005 |
| T4 tumors + unmarried status | 0.481 (0.325–0.710) | <0.001 |
| T4 tumors + positive CEA + left tumors | 0.418 (0.234–0.745) | 0.003 |
| T4 tumors + left tumors + unmarried | 0.547 (0.337–0.794) | 0.002 |
| T4 tumors + <12 LN examined + unmarried status | 0.265 (0.114–0.619) | 0.002 |
| Poorly/undifferentiated tumors + T4 tumors | 0.671 (0.382–0.879) | 0.030 |
| Poorly/undifferentiated tumors + unmarried status | 0.386 (0.171–0.873) | 0.022 |
HR, hazard ratio; HRF, high-risk factor; CI, confidence interval; CEA, carcinoembryonic antigen; PNI, perineural invasion; TD, tumor deposits; LN, lymph nodes.
Establishment and validation of the nomogram
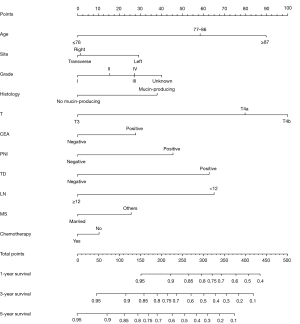
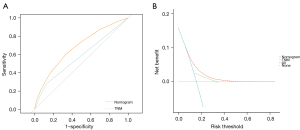
A nomogram model was developed by all the identified HRFs. Figure 3 showed the nomogram model of 1-, 3- and 5-year OS rate. The assessment was internally verified by 1,000 bootstraps, measured by C-index and calibration plots. Specifically, C-index of OS nomogram was 0.704 (95% CI: 0.698–0.716). Meanwhile, the calibration curve showed a high agreement between predictions and actual observations of OS nomogram (Figure 4A-4C). Then, to further compare the nomogram with AJCC TNM stage: in ROC, nomogram (AUC =0.738) in OS showed a larger area than AJCC TNM stage (AUC =0.714) (Figure 5A); in DCA, nomogram in OS showed superior power than AJCC TNM stage (Figure 5B). In summary, nomogram showed better predictive ability and stability compared with AJCC TNM stage.

Discussion
Several previous retrospective studies and randomized controlled trials have indicated that the OS of stage II colon cancer could not benefit from adjuvant chemotherapy (3-5). However, these studies only have analyzed OS or disease-free survival (DFS) generally, ignoring the effect of other treatments on OS, as well as the interactions and differences among the various subgroups of stage II colon cancer. Recently, some studies have found that stage II colon cancer with HRFs can benefit from adjuvant chemotherapy (6,7,11). However, knowledge of HRFs is relatively limited, and some newly discovered HRFs affecting OS, such as tumor location, mucin-producing tumors, tumor deposition, etc., have not been included. Meanwhile, most of these studies have not quantified the amount of risk associated with any specific HRF and assessed the survival benefit conferred by receipt of adjuvant chemotherapy based on the HRFs (7,11,12). Our study, based on SEER database, comprehensively identified most independent risk factors associated with the OS of stage II colon cancer, and included all these independent risk factors into the HRF groups, and then analyzed the HRFs and their combinations in depth. But the limitation in our study was that details (such as chemotherapy regimens, treatment courses, microsatellite instability, etc.) were not available in the SEER database, and some meaningful subgroups may not have been identified.
In our study, T4b tumors were identified as the highest risk for reduced OS, mucin-producing tumors the second, and LN examined less than 12 the third. The benefit of adjuvant chemotherapy among patients with stage II colon cancer was limited to those with specific HRFs. Only T4 tumors, poorly differentiated tumors and some combinations containing either could benefit from chemotherapy. This is basically consistent with the results of a retrospective study based on the California Cancer Registry (CCR) and the study conducted by Kumar et al. (6,13). However, their research mainly reported that benefit was mainly seen in the subgroup of patients with T4 tumors. The difference is that we have find poorly differentiated tumors can also benefit from chemotherapy, and our research have a larger amount of data, while excluding the effect of radiotherapy. In addition, a randomized controlled trial led by Ueno also confirmed our view. The study found that the 5-year RFS rate of patients with poorly differentiated tumors in the chemotherapy group achieved greater improvement (9.1%) than the surgery-alone group (14). Positive CEA, PNI, LN examined less than 12, unmarried status, mucin-producing tumors and left tumors were associated with significant increased risk of mortality compared with patients with no HRFs, and there was a cumulative effect of HRF on OS. Some earlier studies support our results (15-18). However, we found that adjuvant chemotherapy does not significantly improve the survival of patients with these HRFs. On the contrary, a large NCDB study reported that all patients with stage II colon cancer gained benefit from adjuvant chemotherapy regardless of high-risk pathologic features (19). But, the limitation of data variables and the selection bias of the inclusion criteria have led to controversial conclusions.
The general clinical prediction system uses uniform pathological characteristics to predict OS risk, and does not consider the individual differences of patients and the interactions among various risk factors. Meanwhile, due to the long survival of stage II colon cancer, there are many uncertain factors in the follow-up process, which is the limitation of the traditional prediction system. The nomogram model is a visualization tool that comprehensively considers various risk factors affecting OS. It can provide more accurate prediction results for specific patients, possess rich clinical application value, and is gradually adopted by clinicians. The prediction accuracy and discriminative ability of the nomogram have been well verified by the C-index and DCA.
Our study screened all HRFs and quantified the relative risk of death for each HRF in patients with stage II colon cancer. And we found that the survival benefit of adjuvant chemotherapy is limited to some subgroups of patients with T4 tumors, poorly differentiated tumors, and some of their combinations. Meanwhile, we developed high-efficiency prognosis models. We believe these results can help clinicians make adjuvant treatment decisions for high-risk stage II colon cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1779/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1779/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683-91. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Meyers BM, Cosby R, Quereshy F, et al. Adjuvant Chemotherapy for Stage II and III Colon Cancer Following Complete Resection: A Cancer Care Ontario Systematic Review. Clin Oncol (R Coll Radiol) 2017;29:459-65. [Crossref] [PubMed]
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356-63. [Crossref] [PubMed]
- Kumar A, Kennecke HF, Renouf DJ, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer 2015;121:527-34. [Crossref] [PubMed]
- Benson AB 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408-19. [Crossref] [PubMed]
- Marshall JL. Risk assessment in Stage II colorectal cancer. Oncology (Williston Park) 2010;24:9-13. [PubMed]
- Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev 1999;8:1117-21. [PubMed]
- Hayat MJ, Howlader N, Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007;12:20-37. [Crossref] [PubMed]
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797-806. [Crossref] [PubMed]
- Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9. [Crossref] [PubMed]
- Babcock BD, Aljehani MA, Jabo B, et al. High-Risk Stage II Colon Cancer: Not All Risks Are Created Equal. Ann Surg Oncol 2018;25:1980-5. [Crossref] [PubMed]
- Ueno H, Ishiguro M, Nakatani E, et al. Optimal Criteria for G3 (Poorly Differentiated) Stage II Colon Cancer: Prospective Validation in a Randomized Controlled Study (SACURA Trial). Am J Surg Pathol 2020;44:1685-98. [Crossref] [PubMed]
- Amri R, England J, Bordeianou LG, et al. Risk Stratification in Patients with Stage II Colon Cancer. Ann Surg Oncol 2016;23:3907-14. [Crossref] [PubMed]
- Dougaz W, Bouasker I, Gouta EL, et al. Predictive factor of recurrence after curative resection for stage I-II colon cancer. Tunis Med 2019;97:685-91. [PubMed]
- Wang L, Hirano Y, Ishii T, et al. Left colon as a novel high-risk factor for postoperative recurrence of stage II colon cancer. World J Surg Oncol 2020;18:54. [Crossref] [PubMed]
- Spindler BA, Bergquist JR, Thiels CA, et al. Incorporation of CEA Improves Risk Stratification in Stage II Colon Cancer. J Gastrointest Surg 2017;21:770-7. [Crossref] [PubMed]
- Casadaban L, Rauscher G, Aklilu M, et al. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer 2016;122:3277-87. [Crossref] [PubMed]


