
Undifferentiated pleomorphic sarcoma of the extremity and trunk: a retrospective cohort study of 166 cases in a large institution
Introduction
Soft tissue sarcoma (STS) is a type of malignant tumor that develops from mesenchymal tissue, which is estimated to be 1–2% of all malignant tumors, and contains more than 50 histological subtypes (1,2). Undifferentiated pleomorphic sarcoma (UPS) is one of the most common subtypes of STS, previously known as malignant fibrous histiocytoma (MFH) (1,3,4). In 1964 and 1978, O’Brien & Stout and Weiss et al. (5,6) studied the features of MFH and for the first time and then revealed transitions from an area of extremely arranged storiform pattern to a less differentiated area with a pleomorphic appearance. In 2002, the World Health Organization (WHO) reconsidered the definition of MFH, and pointed out that it should be a diagnosis of exclusion. In this view, the term ‘malignant fibrous histiocytoma’ was exchanged by the UPS (7). UPS should be labeled as the exclusion of particular directions of differentiation (8-10), along with the key element which is composed of several types of pleomorphic sarcoma cells with heterogeneity (7). As the most common histological subtype in STS (11), once a clear direction of differentiation can be ruled out, the diagnosis must be considered first in the STSs.
The deep-seated, aggressive and enlarged progressively without pain, always represents the clinical manifestations of STS, and 60–70% occurs in the extremity (3). In addition, approximately 19% of STS originate in the trunk wall (12).
Related reports suggest that the recurrence rate of UPS is greater than 31% (13). Compared with other STSs, the 5-year overall survival (OS) rate is lower in UPS, around 50–70% (9,14-16), and some studies revealed that the 5-year OS rate could be 72% (17). Surgical treatment is largely followed in the UPS (16,18), which can achieve significantly local control for primary UPS. Forty percent of patients with these tumors develop pulmonary metastases (15), which was with 8–12 months of median survival (19).
The current mainstay of treatment for STS is wide resection. And radiotherapy and chemotherapy are effective and recommended adjuvant therapy, its curative effect is not very satisfactory (18,20-22). This research work mainly focuses on the analysis of clinical and pathological features of UPS to confirm the prognostic factors correlated with the OS, metastatic survival and local survival. We aimed to provide the risk factors regarding the survivals in patients with UPS at trunk and extremity. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1795/rc).
Methods
Basic information
One hundred and sixty-six UPS patients (AJCC II and III in trunk and extremity) were included in the existing study, who underwent surgical treatment at the Cancer Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College from January 2005 to January 2018. Detailed clinical features were carefully collected and classified, and the main clinical features include epidemiological statistics of UPS (gender and age of onset), tumor-associated data [site of the tumor, local recurrence (LR) at diagnosis, tumor size, AJCC stage, and resection quality, etc.] and treatment methods (surgery, adjuvant chemotherapy, and adjuvant radiotherapy). The 8th edition AJCC staging for STS of the trunk/extremities divides T-stage into 4 categories and upstages nodal disease to stage IV (23).
The patient’s age was recorded at the moment when the initial diagnosis was carried out in our hospital. The tumor size was the largest diameter of the tumor, and the data comes from pathological results or imaging data. Resection quality were classified into R0 and R1/R2. R0, referred to microscopic tumor-negative surgical margins; R1, referred to microscopic tumor-positive surgical margins; and R2, referred to macroscopic tumor-positive surgical margins.
All histopathological specimens of 166 patients were confirmed by two pathologists. UPS usually appears as isolated, leaf-like, or fish-like masses, and the cut surface is commonly white or gray. Under hematoxylin-eosin (HE) staining, it mostly appears as a mixed growth pattern of matted areas and polymorphic areas with a large number of polymorphic areas. The chromatin and irregular nuclei were present in multinucleated giant cells, as depicted in Figure 1.
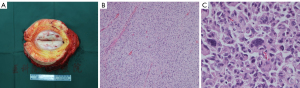
The inclusion/exclusion criteria for patients enrolled in this study were: (I) surgery, must be performed at our hospital; (II) patients who only received radiotherapy and/or chemotherapy were excluded; (III) the pathological diagnosis, as well as the surgical margins, must be determined by our pathology department; and (IV) long-term follow-up data must be detailed and reliable completely.
The study was established, according to the ethical guidelines of the Helsinki Declaration (as revised in 2013) and was approved by the Human Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital (No. NCC2020C-341). Written informed consent was obtained from individual or guardian participants.
Treatment and follow-up data
The resection quality of all patients was evaluated at our hospital, and those cases who just received chemotherapy and/or radiotherapy were excluded. Postoperative pathological indications were as follows; 90.4% (150/166) and 9.6% (16/166) patients were R0 and R1/R2 resection, respectively. For patients with high recurrence rate and/or high metastasis rate, we generally recommend radiotherapy and/or chemotherapy. Fifty point six percent (84/166) patients underwent adjuvant radiation therapy in the period of the disease, with an average radiotherapy dose of 50 Gy (15–76 Gy) and a median radiation dose of 60 Gy. Thirty-three point seven percent (56/166) patients received adjuvant chemotherapy. Ifosfamide and doxorubicin were mainly used as chemotherapeutic drugs. The regular checkups including regular chest CT and local MRI scans were carried out post operation in our hospital. Follow-up data were collected by phone calls and medical records. The follow-up time of 166 patients was 6–168 months, with a mean follow-up time of 62 months and a median follow-up of 55 months.
Statistical analysis
The statistical analysis was carried out by SPSS 22.0 and GraphPad Prism 6. While the Kaplan-Meier method and Cox regression model were employed for univariate and multivariate analysis. P value less than 0.05 was regarded as statistically considerable.
Results
Among them, male patients and female patients were 54.2% (90/166) and 45.8% (76/166), respectively and their ages were 24 to 83 years with median and the average age of 57 and 55.5 years, respectively. The UPS in the trunk, upper extremity and lower extremity accounted for 30.1% (50/166), 15.7% (26/166), and 54.2% (90/166), respectively. Patients with no recurrence tumors and recurrent tumors accounted for 62.7% (104/166) and 37.3% (62/166), respectively. The tumor size ranged from 1 to 22 cm, with an average size of 5.52 cm. The diameter of tumors was 5 cm or less in 57.8% (96/166), while in 42.2% (70/166) the diameter of the tumor was more than 5 cm. According to the American Joint Committee on Cancer (AJCC) staging criteria, stage II and stage III accounted for 57.8% (96/166) and 42.2% (70/166), accordingly, as presented in Table 1.
Table 1
| Variables | Quantity | Percentage (%) |
|---|---|---|
| Demographic characteristics | ||
| Age (years) | ||
| ≤60 | 101 | 60.8 |
| >60 | 65 | 39.2 |
| Gender | ||
| Male | 90 | 54.2 |
| Female | 76 | 45.8 |
| Tumor features | ||
| Tumor site | ||
| Trunk | 50 | 30.1 |
| Upper extremity | 26 | 15.7 |
| Lower extremity | 90 | 54.2 |
| Local recurrence at diagnosis | ||
| No recurrence | 104 | 62.7 |
| Recurrence | 62 | 37.3 |
| Tumor size (cm) | ||
| ≤5 | 96 | 57.8 |
| >5 | 70 | 42.2 |
| Tumor grades | ||
| AJCC grades | ||
| II | 96 | 57.8 |
| III | 70 | 42.2 |
| Pathological features | ||
| Resection quality | ||
| R0 | 150 | 90.4 |
| R1/R2 | 16 | 9.6 |
| Adjuvant treatment | ||
| Radiotherapy | 84 | 50.6 |
| Chemotherapy | 56 | 33.7 |
| Combined | 42 | 25.3 |
| Nil | 62 | 37.3 |
| Prognosis | ||
| Post-treatment local recurrence | 38 | 22.9 |
| Post-treatment metastases | 54 | 32.5 |
| Death | 41 | 24.7 |
UPS, undifferentiated pleomorphic sarcoma.AJCC, American Joint Committee on Cancer.
LR
At the end of follow-up, 22.9% (38/166) was the LR rate of 166 UPS patients with a median follow-up of 55 months. The 3- and 5-year LR-free survival (LRFS) rate were 79.2% and 74.4%, respectively (Figure 2A). Factors influencing LRFS in univariate analyses and multivariate analysis were listed in Table 2. Univariate analysis revealed that the significant factors correlated with higher LR rate were female, recurrence patients and R1/R2 (Table 2, Figure 2B-2D). The multivariate analysis revealed that gender (P=0.008, HR =0.410, 95% CI: 0.212–0.796) and resection quality (P=0.001, HR =3.626, 95% CI: 1.675–7.846) were two independent risk factors for LR in patients with UPS post operation (P<0.05), which was presented in Table 2. The female patients had a 1.92-fold increased risk of developing LR than male patients (HR =2.285, 95% CI: 1.213–4.383, P=0.0111), as depicted in Figure 2B. With respect to the resection quality, a considerable variation was found between the two groups for LRFS (HR =3.758, 95% CI: 3.064–33.63, P=0.0002), as given in Figure 2D. R1/R2 resection margins had a high LR rate in UPS (24), which was confirmed again by us. Radiotherapy is an important means to control tumor recurrence after surgery. In this article, it is not found that radiotherapy is meaningful for the control of postoperative recurrence of UPS.
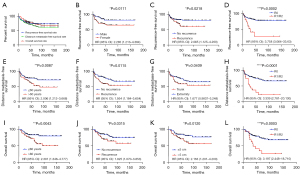
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| 3-year LRFS rate | 5-year LRFS rate | P value | HR | 95% CI | P value | ||
| Gender | 0.011 | 0.008 | |||||
| Male | 85.5 | 81.9 | 0.410 | 0.212–0.796 | |||
| Female | 71.5 | 65.3 | |||||
| Age (years) | 0.113 | – | |||||
| ≤60 | 82.8 | 79.5 | – | – | |||
| >60 | 73.6 | 66.3 | – | – | |||
| Local recurrence at diagnosis | 0.022 | 0.076 | |||||
| No recurrence | 83.3 | 81.6 | 1.800 | 0.939–3.450 | |||
| Recurrence | 72.2 | 61.2 | |||||
| Tumor sites | 0.696 | – | |||||
| Trunk | 83.7 | 76.7 | – | – | |||
| Extremity | 77.5 | 73.4 | – | – | |||
| Tumor size (cm) | 0.183 | – | |||||
| ≤5 | 81.7 | 78.8 | – | – | |||
| >5 | 75.7 | 67.5 | – | – | |||
| AJCC grade | 0.183 | – | |||||
| II | 81.7 | 78.8 | – | – | |||
| III | 75.7 | 67.5 | – | – | |||
| Resection quality | 0.000 | 0.001 | |||||
| R0 | 82.7 | 78.5 | 3.626 | 1.675–7.846 | |||
| R1/R2 | 41.3 | 27.6 | |||||
| Adjuvant radiotherapy | 0.329 | – | |||||
| Yes | 81.1 | 78.9 | – | – | |||
| No | 77.3 | 70.1 | – | – | |||
| Adjuvant chemotherapy | 0.221 | – | |||||
| Yes | 74.4 | 68.3 | – | – | |||
| No | 81.8 | 77.5 | – | – | |||
LRFS, local recurrence-free survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer.
Distant metastasis (DM)
In 166 UPS patients, the rate of DM was 32.5% (54/166). The 3- and 5-year distant metastasis-free survival (DMFS) rates were 74.5% and 67.6%, respectively (Figure 2A). In the existing study, univariate analysis revealed that prognostic factors i.e., older (>60 years), LR at diagnosis, trunk and R1/R2 had considerable variations in DMFS (Table 3, Figure 2E-2H). The significant results of the univariate analysis were incorporated into the cox multivariate analysis, and then we revealed the independent factors i.e., older (>60 years) (P=0.044, HR =1.780, 95% CI: 1.016–3.116), trunk (P=0.002, HR =0.396, 95% CI: 0.219–0.718), R1/R2 (P=0.006, HR =2.566, 95% CI: 1.315–5.005), and adjuvant chemotherapy (P<0.001, HR =2.992, 95% CI: 1.666–5.371) had a more possibility of DM (all P<0.05), as presented in Table 3.
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| 3-year DMFS rate | 5-year DMFS rate | P value | HR | 95% CI | P value | ||
| Gender | 0.695 | – | |||||
| Male | 70.6 | 65.9 | – | – | |||
| Female | 78.5 | 69.6 | – | – | |||
| Age (years) | 0.009 | 0.044 | |||||
| ≤60 | 78.1 | 76.3 | 1.780 | 1.016–3.116 | |||
| >60 | 68.9 | 52.4 | |||||
| Local recurrence at diagnosis | 0.012 | 0.091 | |||||
| No recurrence | 82.5 | 77.3 | 1.603 | 0.928–2.769 | |||
| Recurrence | 61.3 | 56.8 | |||||
| Tumor site | 0.041 | 0.002 | |||||
| Trunk | 61.4 | 58.8 | 0.396 | 0.219–0.718 | |||
| Extremity | 80.0 | 71.5 | |||||
| Tumor size (cm) | 0.070 | 0.386 | |||||
| ≤5 | 80.1 | 75.4 | 1.276 | 0.736–2.213 | |||
| >5 | 66.7 | 57.0 | |||||
| AJCC grade | 0.070 | – | |||||
| II | 80.1 | 75.4 | – | – | |||
| III | 66.7 | 57.0 | – | – | |||
| Resection quality | 0.001 | 0.006 | |||||
| R0 | 78.5 | 73.0 | 2.566 | 1.315–5.005 | |||
| R1/R2 | 37.5 | 22.5 | |||||
| Adjuvant radiotherapy | 0.863 | – | |||||
| Yes | 74.7 | 68.0 | – | – | |||
| No | 74.2 | 67.4 | – | – | |||
| Adjuvant chemotherapy | 0.001 | <0.001 | |||||
| Yes | 79.8 | 75.8 | 2.992 | 1.666–5.371 | |||
| No | 64.2 | 52.5 | |||||
DMFS, distant metastasis-free survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer.
OS
The OS rate of 166 UPS patients was 75.3% (125/166) until the end of follow-up, while the 3- and 5-year OS rates were 81.7% and 76.4%, respectively. In our study, univariate analysis reveals that prognostic factors i.e., older (>60 years), LR at diagnosis, tumor size (>5 cm), AJCC stage (III) and R1/R2 were considerably associated with the poor OS rate (P<0.05) (Table 4, Figure 2I-2L). The effective results of the univariate analysis were incorporated into the cox multivariate analysis which confirms the three independent factors i.e., trunk (P=0.047, HR =0.526, 95% CI: 0.279–0.992), R1/R2 (P<0.001, HR =3.742, 95% CI: 1.853–7.554) and tumor size (>5 cm) (P=0.022, HR =2.093, 95% CI: 1.110–3.944) which correlated with a poorer OS, as presented in Table 4. In this study, in 54 patients with metastases, whether chemotherapy or not was significantly related to overall prognosis.
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| 3-year OS rate | 5-year OS rate | P value | HR | 95% CI | P value | ||
| Gender | 0.758 | – | |||||
| Male | 84.2 | 77.3 | – | – | |||
| Female | 81.5 | 75.0 | – | – | |||
| Age (years) | 0.004 | – | |||||
| ≤60 | 86.1 | 83.8 | – | – | |||
| >60 | 78.2 | 65.5 | – | – | |||
| Local recurrence at diagnosis | 0.031 | – | |||||
| No recurrence | 86.4 | 83.5 | – | – | |||
| Recurrence | 74.1 | 65.0 | – | – | |||
| Tumor site | 0.128 | 0.047 | |||||
| Trunk | 75.3 | 72.4 | 0.526 | 0.279–0.992 | |||
| Extremity | 84.3 | 80.9 | |||||
| Tumor size (cm) | 0.012 | 0.022 | |||||
| ≤5 | 85.2 | 83.4 | 2.093 | 1.110–3.944 | |||
| >5 | 76.9 | 66.7 | |||||
| AJCC grade | 0.012 | – | |||||
| II | 85.2 | 83.4 | – | – | |||
| III | 76.9 | 66.7 | – | – | |||
| Resection quality | <0.001 | <0.001 | |||||
| R0 | 84.5 | 81.3 | 3.742 | 1.853–7.554 | |||
| R1/R2 | 56.3 | 35.2 | |||||
| Adjuvant radiotherapy | 0.843 | – | |||||
| Yes | 82.1 | 77.0 | – | – | |||
| No | 81.2 | 75.9 | – | – | |||
| Adjuvant chemotherapy | 0.226 | – | |||||
| Yes | 84.4 | 79.0 | – | – | |||
| No | 76.6 | 71.5 | – | – | |||
OS, overall survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer.
Discussion
UPS, called MFH previously, which was recognized as the most common STS in adults, accounting for 50% of diagnoses. However, the pathological diagnosis of UPS shown no evidence of true histiocytic differentiation, meaning it encompasses the morphologic manifestations of a variety of poorly differentiated tumors rather than being a single entity (15). So the diagnosis and treatment of UPS are still highly challenging because of the confused pathological classification. MRI is commonly used as a non-invasive effective diagnostic tool for STS.
R1/R2 resection margins identified as predictors of poor outcomes. Herein, the R0 resection margin was an only independent favorable prognostic factor that was correlated with LRFS, DMFS, and OS. The resection margin was found to be the prognostic factor that was effectively correlated with the duration of survival. Peiper et al. (13) proposed that positive microscopic margins were correlated with an elevated LR rate (RR =4.8, P<0.01). Özkurt et al. (25) studied 14 cases of confirmed bone UPS and it was found that the 5-year survival rate of patients with wide resection and border resection were 81.9% and 33.3% (P<0.05), which reveals that surgical excision with wide margins and adjuvant chemotherapy provided adequate control of the disease and longer survival. Just like some article says that surgery striving for negative margins, with radiotherapy, is the treatment of choice (10,15,24).
With respect to tumor size, Winchester et al. (26) evaluated the prognostic factors of 319 UPS patients and revealed that tumor size (greater than 5 cm) and deep subcutaneous fat infiltration were significant factors that affect the LR rate. In the existing study, compared with those with tumor sizes ≤5 and >5 cm, the 5-year LR, DM and OS rates decreased by 11.3%, 18.4% and 16.7%, respectively (Table 2). The extensive analysis of the data of more cases may contribute to better resolve the underlined problem.
The metastasis predominantly occurs in the lungs (10,27) relative to regional lymph nodes (28). Winchester et al. (26) suggested that the main factors that affect the DM of UPS were the tumor site, tumor size larger than 2 cm, invasion beyond subcutaneous fat, and lymphovascular invasion. In the existing study, cox multivariate survival analysis found that >60 years were at a higher risk of metastasis than the younger patients, and the chances of metastasis were lower in the R0 resection margin, as presented in Table 3. Furthermore, in multivariate analysis, the tumor site was an independent predictor correlated with DMFS, as depicted in Figure 2F. Our findings of increased metastatic disease for the UPS in trunk is likely due to trunk tumors being more possibility and visible to hematogenous metastasis in the early stages of disease.
In the analysis of OS, the Cox multivariate survival analysis revealed that tumor site (P=0.026), tumor size (P=0.048), AJCC stage (P=0.048), and resection quality (P=0.001) were independent factors that affect postsurgical survival in UPS patients (all P<0.05), as represented in Table 4. According to our cohort, for the patients having tumors of the trunk, the tumor size ≥5 cm and R1/R2, a more significant and effective approach should be adopted. Winchester et al. (26) found that age, immunosuppression, tumor size larger than 2 cm, and lymphovascular invasion were independent risk factors affecting overall prognosis. Simultaneously, the existing study revealed that patients with severe subcutaneous fatty infiltration of tumors had a bad prognosis rate. In the AJCC staging system, tumor size and tumor depth were significantly associated with the prognosis.
In the AJCC staging guidelines, tumor size is an important criteria for the judgment of soft tissue staging. Univariate analysis revealed that the size of the tumor was not considerably associated with LRFS and DMFS, but was closely associated with OS (P=0.012), as shown in Figure 2K. In multivariate analysis, tumor size (≥5 cm) was not an independent prognostic factor affecting LRFS and DMFS (all P>0.05). Furthermore, in 2009, Lehnhardt et al. (17) also shown that tumor size ≥5 cm was considerably associated with the OS, which was in line with Chen and Al-Agha (27,29). Our study also confirmed that tumor size ≥5 cm was also one of the most important factors affecting OS. Peiper et al. (13) found that tumor size (RR =6.0, P<0.01) was a significant factor that affects the DFS of UPS patients. Larger tumors suggest a higher ability to divide and proliferate, a wider range of invasion, a higher degree of malignancy, and more complicated surgical methods, so the first visit to the professional sarcoma center is critical.
In the existing study, Univariate K-M analysis revealed that the LR at diagnosis was a significant factor that affects the LR rate, DM rate, and OS rate (P<0.05). But in multivariate analysis, the presentation of tumor was not an independent prognostic factor affecting LR rate, DM rate, and OS rate, with P values of 0.076, 0.091, and 0.162, respectively. Lehnhardt et al. (17) shown that a considerable variation was found between the group presenting with primary tumors (5-year survival: 84%, P<0.05) and recurrent tumors (5-year survival: 62%, P<0.05), which is correlated with our existing research work. The prognosis for patients with UPS of the extremities depends predominantly on adequate wide resection of the primary tumor, which is same to the idea that complete surgical resection was the most important UPS treatment strategy for UPS (18). In short, the LR at diagnosis and then R0 resection in the first therapy may play a crucial role in patient prognosis.
The value of adjuvant radiotherapy and chemotherapy in the diagnosis and treatment of STS has been mixed. Radiotherapy is mostly considered to be an effective mean to control local tumor recurrence, but in this study, it was not found that radiotherapy has any significance in the control of UPS. Trials from Gronchi suggested an OS benefit with five cycles of adjuvant full-dose epirubicin plus ifosfamide in localised high-risk soft-tissue sarcoma of the extremities or trunk wall (30,31). Adjuvant chemotherapy was associated with improved LRFS only in patients ≥30 years (32). Pazopanib and immune checkpoint inhibitors are a new attempt in UPS treatment (33-35). UPS is an immunologically active subtype of STS, which is particularly amenable to immune checkpoint inhibitors (35). Immunohistochemical biomarkers significantly contribute to predicting the rate of recurrence, metastasis, and OS rate. A significant predictive index for evaluating the effect of VEGFR receptor inhibitors in the treatment of advanced STS, TP53 plays a significant role in the diagnosis and treatment of UPS (36). Therefore, an extensive study on the molecular mechanism is needed to explore the targeted therapy and feasibility of immune checkpoint inhibitors(18,37-39).
As a retrospective study, although this study has given us a crucial hint, there are some shortcomings in the existing study. Firstly, the statistics on chemotherapy and radiotherapy are not sufficient due to the low incidence rate of the underlined disease, limited samples, and a large time span and the significant evaluation of the adjuvant therapy is also very difficult. Nevertheless, the accumulation and analysis of more comprehensive medical data for UPS can objectively reflect the characteristics and outcome of the disease that needs to be improved.
Conclusions
The existing study determines that the UPS in trunk, tumor size ≥5 cm and R1/R2 resection margin are prognostic markers of poor over survival. R1/R2 resection margin significantly correlated with high LR rate and women are more susceptible to LR. The UPS in trunk and R1/R2 resection margin are significantly correlated with DM and old patients (>60 years) are more susceptible to DM.
Acknowledgments
We appreciated all the UPS patients in this article.
Funding: CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2017-I2M-1-005); Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2016L01); Capital Characterized Clinical Application Research Fund of Beijing Municipal Science and Technology Commission of China (No. Z171100001017210); Clinical Research Special Fund of Wu Jieping Medical Foundation (No. 320.6750.14298)
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1795/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1795/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1795/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was established, according to the ethical guidelines of the Helsinki Declaration (as revised in 2013) and was approved by the Human Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital (No. NCC2020C-341). Written informed consent was obtained from individual or guardian participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singer S, Demetri GD, Baldini EH, et al. Management of soft-tissue sarcomas: an overview and update. Lancet Oncol 2000;1:75-85. [Crossref] [PubMed]
- Penel N, Coindre JM, Giraud A, et al. Presentation and outcome of frequent and rare sarcoma histologic subtypes: A study of 10,262 patients with localized visceral/soft tissue sarcoma managed in reference centers. Cancer 2018;124:1179-87. [Crossref] [PubMed]
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and high-grade undifferentiated pleomorphic sarcoma-what’s in a name? J Surg Oncol 2015;111:173-7. [Crossref] [PubMed]
- Matushansky I, Charytonowicz E, Mills J, et al. MFH classification: differentiating undifferentiated pleomorphic sarcoma in the 21st Century. Expert Rev Anticancer Ther 2009;9:1135-44. [Crossref] [PubMed]
- O’Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer 1964;17:1445-55. [Crossref] [PubMed]
- Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer 1978;41:2250-66. [Crossref] [PubMed]
- Fletcher CD. The evolving classification of soft tissue tumours—an update based on the new 2013 WHO classification. Histopathology 2014;64:2-11. [Crossref] [PubMed]
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014;46:95-104. [Crossref] [PubMed]
- Lewin J, Garg S, Lau BY, et al. Identifying actionable variants using next generation sequencing in patients with a historical diagnosis of undifferentiated pleomorphic sarcoma. Int J Cancer 2018;142:57-65. [Crossref] [PubMed]
- Chamale JB, Bruno M, Mandojana F, et al. Malignant fibrous histiocytoma in the right portion of the mandible with metastasis in pancreas. Int J Surg Case Rep 2017;41:71-5. [Crossref] [PubMed]
- Boccalatte LA, Gómez NL, Yanzon A, et al. Head and neck tumors: management of primary undifferentiated pleomorphic sarcoma. Iran J Otorhinolaryngol 2019;31:335-42. [PubMed]
- Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94-109. [Crossref] [PubMed]
- Peiper M, Zurakowski D, Knoefel WT, et al. Malignant fibrous histiocytoma of the extremities and trunk: an institutional review. Surgery 2004;135:59-66. [Crossref] [PubMed]
- Beck AH, West RB, van de Rijn M. Gene expression profiling for the investigation of soft tissue sarcoma pathogenesis and the identification of diagnostic, prognostic, and predictive biomarkers. Virchows Arch 2010;456:141-51. [Crossref] [PubMed]
- Vodanovich DA, Spelman T, May D, et al. Predicting the prognosis of undifferentiated pleomorphic soft tissue sarcoma: a 20-year experience of 266 cases. ANZ J Surg 2019;89:1045-50. [Crossref] [PubMed]
- Malik AT, Baek J, Alexander JH, et al. Malignant fibrous histiocytoma of bone: a survival analysis from the National Cancer Database. J Surg Oncol 2020;121:1097-103. [Crossref] [PubMed]
- Lehnhardt M, Daigeler A, Homann HH, et al. MFH revisited: outcome after surgical treatment of undifferentiated pleomorphic or not otherwise specified (NOS) sarcomas of the extremities -- an analysis of 140 patients. Langenbecks Arch Surg 2009;394:313-20. [Crossref] [PubMed]
- Lee K, Song JS, Kim JE, et al. The clinical outcomes of undifferentiated pleomorphic sarcoma (UPS): a single-centre experience of two decades with the assessment of PD-L1 expressions. Eur J Surg Oncol 2020;46:1287-93. [Crossref] [PubMed]
- Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol 2003;21:2719-25. [Crossref] [PubMed]
- Ibanez MA, Rismiller K, Knackstedt T. Prognostic factors, treatment, and survival in cutaneous pleomorphic sarcoma. J Am Acad Dermatol 2020;83:388-96. [Crossref] [PubMed]
- Gronchi A, Palmerini E, Quagliuolo V, et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) sarcoma groups. J Clin Oncol 2020;38:2178-86. [Crossref] [PubMed]
- Goertz O, Pieper A, Lohe LV, et al. The impact of surgical margins and adjuvant radiotherapy in patients with undifferentiated pleomorphic sarcomas of the extremities: a single-institutional analysis of 192 patients. Cancers (Basel) 2020;12:362. [Crossref] [PubMed]
- Fisher SB, Chiang YJ, Feig BW, et al. Comparative performance of the 7th and 8th editions of the american joint committee on cancer staging systems for soft tissue sarcoma of the trunk and extremities. Ann Surg Oncol 2018;25:1126-32.
- Kamat NV, Million L, Yao DH, et al. The outcome of patients with localized undifferentiated pleomorphic sarcoma of the lower extremity treated at Stanford University. Am J Clin Oncol 2019;42:166-71. [Crossref] [PubMed]
- Özkurt B, Başarır K, Yıldız YH, et al. Primary malignant fibrous histiocytoma of long bones: long-term follow-up. Eklem Hastalik Cerrahisi 2016;27:94-9. [Crossref] [PubMed]
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: Factors predictive of adverse outcomes. J Am Acad Dermatol 2018;79:853-9. [Crossref] [PubMed]
- Chen S, Huang W, Luo P, et al. Undifferentiated pleomorphic sarcoma: long-term follow-up from a large institution. Cancer Manag Res 2019;11:10001-9. [Crossref] [PubMed]
- Senel FC, Bektas D, Caylan R, et al. Malignant fibrous histiocytoma of the mandible. Dentomaxillofac Radiol 2006;35:125-8. [Crossref] [PubMed]
- Al-Agha OM, Igbokwe AA. Malignant fibrous histiocytoma: between the past and the present. Arch Pathol Lab Med 2008;132:1030-5. [Crossref] [PubMed]
- Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812-22. [Crossref] [PubMed]
- Gronchi A, Stacchiotti S, Verderio P, et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann Oncol 2016;27:2283-8. [Crossref] [PubMed]
- Kasper B, Ouali M, van Glabbeke M, et al. Prognostic factors in adolescents and young adults (AYA) with high risk soft tissue sarcoma (STS) treated by adjuvant chemotherapy: a study based on pooled European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62771 and 62931. Eur J Cancer 2013;49:449-56. [Crossref] [PubMed]
- Kim HJ, Kim Y, Lee SJ, et al. Pazopanib monotherapy in the treatment of pretreated, metastatic uterine sarcoma: a single-center retrospective study. J Gynecol Oncol 2018;29:e3. [Crossref] [PubMed]
- Guram K, Nunez M, Einck J, et al. Radiation therapy combined with checkpoint blockade immunotherapy for metastatic undifferentiated pleomorphic sarcoma of the maxillary sinus with a complete Response. Front Oncol 2018;8:435. [Crossref] [PubMed]
- Arora S, Rastogi S, Shamim SA, et al. Good and sustained response to pembrolizumab and pazopanib in advanced undifferentiated pleomorphic sarcoma: a case report. Clin Sarcoma Res 2020;10:10. [Crossref] [PubMed]
- Movva S, Wen W, Chen W, et al. Multi-platform profiling of over 2000 sarcomas: identification of biomarkers and novel therapeutic targets. Oncotarget 2015;6:12234-47. [Crossref] [PubMed]
- Monga V, Skubitz KM, Maliske S, et al. A retrospective analysis of the efficacy of immunotherapy in metastatic soft-tissue sarcomas. Cancers (Basel) 2020;12:1873. [Crossref] [PubMed]
- Miyake M, Oda Y, Nishimura N, et al. Integrative assessment of clinicopathological parameters and the expression of PD-L1, PD-L2 and PD-1 in tumor cells of retroperitoneal sarcoma. Oncol Lett 2020;20:190. [Crossref] [PubMed]
- Keung EZ, Burgess M, Salazar R, et al. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin Cancer Res 2020;26:1258-66. [Crossref] [PubMed]


