
Comparison of the safety and efficacy of hepatic resection and radiofrequency ablation in the treatment of single small hepatocellular carcinoma: systematic review and meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide (1) Among other malignancies, the incidence of HCC ranks fifth in men, ninth in women, and second in global cancer deaths (2). The national cost of HCC hospitalization in the United States increased from 241 million US dollars in 1988 to 509 million US dollars in 2000. HCC not only seriously affects the lives and health of people around the world, but also results in a huge burden to people’s lives. With the increasing health needs of the population, more attention is urgently needed to understand the risk factors of HCC and implement preventive measures (3). During the diagnosis and treatment patients with HCC, attention should be paid to adopting a multidisciplinary diagnostic and treatment model, so as to avoid the limitations of a single treatment modality (4).
For patients with early-stage tumors, resection, liver transplantation and radiofrequency ablation (RFA) are curable treatments, which may lead to a 5-year survival rate of approximately 50% (5). Hepatic resection (HR) and liver transplantation are considered to be effective methods for the treatment of HCC; however, most patients with liver cancer have lost the opportunity for surgical resection due to complicated liver cirrhosis and multiple tumors (6,7). The technique of liver transplantation has been severely undermined by the scarcity of donor organs, while hepatectomy remains the first-line treatment for HCC. However, due to poor hepatic reserve secondary to chronic liver disease or the multifocal distribution of tumor nodules, only 9–29% of patients are eligible for HR (8,9).
With the development of science and technology, as well as the popularization of minimally invasive technology, local ablation represented by microwave and RFA technology has been widely applied in the treatment of HCC (10-12). RFA has the advantages of small trauma, high safety, and reproducibility, and is widely used in the clinical treatment of HCC, especially for single small HCC, which can achieve the goal of complete radical cure (13,14), and has been considered as the third treatment option for liver cancer after HR and interventional therapy (15).
In recent years, minimally invasive HR has developed rapidly, but HR and RFA are still controversial in the treatment of single small HCC at home and abroad. Huang and his colleagues (16) report that HR has more advantages (survival and recurrence rates), regardless of tumor size (greater than or less than 3 cm; even less than 2 cm), in addition, Vivarelli and colleagues (17) believe that RFA is as effective as HR in treating small HCC alone. Therefore, this study compared the efficacy of HR and RFA in the treatment of single small HCC through meta-analysis and systematic evaluation, which provided a reference for clinical treatment decisions.
We present the following article in accordance with the MOOSE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-563/rc).
Methods
Literature inclusion and exclusion criteria
Research design
The search time is from the establishment of the database to January 15, 2022. There are studies on hepatectomy and RFA in the treatment of small liver cancer, which are not limited by sample size, language or national region.
Inclusion criteria
- Studies involving patients with single HCC confirmed by imaging or needle biopsy according to the Milan criteria;
- Articles involving patients that were generally in good condition without other organic lesions;
- Studies involving a consistent study baseline of patients;
- Articles involving patients who agreed to be treated with HR or RFA.
- Case control study.
Exclusion criteria
- Conference abstracts, systematic ratings, and repeated publications;
- Studies that did not satisfy the Milan criteria;
- Single studies with a total of less than five cases;
- Articles involving patients with poor liver function, combined with other diseases that affect life expectancy;
- Studies involving simple surgery or RFA treatment.
Interventions
Hepatectomy and RFA were used to treat single small HCC that met the Milan criteria. The study group was treated with RFA and the control group with HR. There was no other adjuvant therapy in either group, such as hepatic artery embolization or drug therapy.
Observed indicators
The 1-, 3-, and 5-year overall survival rates, postoperative complications, intraoperative blood loss, operation time, and recurrence were used as the observed outcome indicators.
Literature retrieval
The PubMed, Embase, and Cochrane Library English databases were searched for clinical studies published between 2010 and January 15, 2022 on RFA and HR in the treatment of single small HCC using search terms such as RFA, HR, small HCC, and single.
Literature data extraction
Two researchers independently screened the literature for data extraction, and performed preliminary screening by reading the titles and abstracts of the retrieved articles. For studies that were easy to evaluate, literature screening was carried out directly; however, for those articles with discrepancies in terms of the inclusion criteria, relevant teachers were consulted for advice, and the full text was downloaded and read directly for screening. In the screening process, the inclusion and exclusion criteria were strictly followed, and two researchers independently extracted the data of each observed indicator of the studies, and cross-checked the extracted data to ensure the consistency of the data from the literature.
Quality evaluation
To assess methodological quality, the two authors jointly evaluated the risk of bias in different studies. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the cohort or case-control studies. The scale consisted of two tables, namely cohort study and case-control study. The cohort study contained eight items from three aspects, including study population selection, inter-group comparability, and outcome measurement, while the case-control study contained eight items from three aspects, including study population selection, inter-group comparability, and exposure measurement. If the requirements were met, one point would be counted, with a maximum score of 9 points; a study with ≥5 points would be regarded as high-quality literature.
Statistical analysis
The extracted data were entered into Review Manager 5.4 software provided by the Cochrane Collaboration for analysis. A heterogeneity test was initially performed on the data. If P>0.1 and I2≤50%, it was considered that there was no statistical heterogeneity, and a fixed-effects model was selected for combined analysis. However, if P<0.1 and I2>50%, it was considered that statistical heterogeneity existed, and a random-effects model was adopted for combined analysis. For obvious heterogeneity, subgroup or sensitivity analyses were used to explore the cause and source of heterogeneity. The weighted mean difference (MD) was used as the statistical effect size for continuous data, and the odds ratio (OR) was used as the statistical effect size for binary data. Both categories of data were expressed using 95% confidence interval (CI). P<0.05 was considered to indicate a statistically significant difference. RevMan 5.4 software was employed to construct funnel plots to qualitatively evaluate the publication bias, with a symmetrical funnel plot suggesting small publication bias. Sensitivity analysis was performed by excluding studies one-by-one to test the robustness of the results.
Results
Literature retrieval process and results
A total of 378 articles were initially retrieved, and 309 were obtained after eliminating duplicates. A further 285 articles were excluded after reading the titles and abstracts, and another 13 were removed due to incomplete results, unavailable data, and low literature quality after reading the full texts. Finally, a total of 11 (18-28) related articles were included, all of which were case-control trials, involving a total of 2,001 patients. The retrieval process is shown in Figure 1.
Basic characteristics and quality evaluation of the included literature
All of the 11 included controlled studies on HR and RFA in the treatment of small HCC were case-control trials with NOS scores ≥6 points. The overall quality of the included literature was high, and the studies were representative to a certain extent. A total of 2,001 patients were enrolled in the 11 studies, including 1,071 patients who underwent RFA and 930 patients who underwent HR. Table 1 shows the basic characteristics of the included literature, and the quality evaluation is presented in Table 2.
Table 1
| Study | Age (year) | Country | Sex (n) | Number of participants | Child-A (n) | Tumor size (cm) | Follow-up (years) | Outcome measures | NOS scores | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | RFA | M | F | HR | RFA | ||||||||
| Cha DI 2020 | 53.3±10 | 56.75±9.5 | Korea | 253 | 70 | 145 | 178 | 131 | <3 | 10 | OS; C | 8 | |
| Di Sandro S 2019 | 66±2.03 | 65.5±4.06 | Italy | 48 | 134 | 91 | 91 | NA | <5 | 5 | OS; TR | 9 | |
| Hsiao CY 2020 | 58.8±11.7 | 62.2±12.3 | China | 236 | 151 | 156 | 231 | NA | <2.5 | 7 | OS; TR | 9 | |
| Kang TW 2015 | 52±8.78 | 56.5±8.38 | Korea | 154 | 45 | 99 | 99 | 78 | <2 | 8 | OS; C; HS | 8 | |
| Kim GA 2016 | 55.4±8.3 | 55.4±10 | Korea | 240 | 64 | 152 | 152 | NA | <3 | 6 | RFS; TR | 8 | |
| Lai C 2016 | 56.5±12.6 | 62.8±11.3 | China | 53 | 8 | 28 | 33 | 57 | <3 | 3 | OT; BL; HS; TR | 9 | |
| Lin CH 2020 | NA | NA | China | 52 | 23 | 36 | 39 | NA | <2 | 6 | OS | 6 | |
| Liu PH 2016 | 60±13 | 64±12 | China | 107 | 51 | 79 | 79 | NA | <2 | 8 | OS; TR | 8 | |
| Song J 2016 | 49.25±2.70 | 49±2.90 | China | 140 | 16 | 78 | 78 | 154 | <4 | 8 | OT; BL; HS; TR; OS | 9 | |
| Vitali GC 2016 | 59.45±12.03 | 66.15±7.78 | Switzerland | 82 | 23 | 45 | 60 | 85 | <4 | 12 | OT; HS; C | 8 | |
| Zhou Z 2014 | 42.2±7.6 | 46.7±9.8 | China | 35 | 17 | 21 | 31 | 26 | <3 | 5 | OT; BL; HS; OS; C | 8 | |
BL, blood loss; C, complications; F, female; HR, hepatic resection; HS, hospital stay; M, male; NA, not mention; NOS, Newcastle-Ottawa Scale; OS, overall survival; OT, operating time; RFA, radiofrequency ablation; TR, tumor recurrence.
Table 2
| Study | A | B | C | D | E | F | G | H | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Cha DI 2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Di Sandro S 2019 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 10 |
| Hsiao CY 2020 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 10 |
| Kang TW 2015 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Kim GA 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Lai C 2016 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 10 |
| Lin CH 2020 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Liu PH 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Song J 2016 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 10 |
| Vitali GC 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhou Z 2014 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
A: definition of cases; B: representativeness of cases; C: selection of controls; D: definition of comparison; E: case and control comparability based on design or analysis; F: determination of exposure; G: whether the same determination method was used for case and control exposures; H: no response rate.
Meta-analysis results
Postoperative 1-year overall survival rate
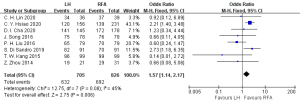
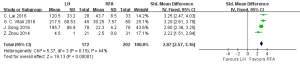
Eight articles (18-21,24-26,28) reported on the postoperative 1-year overall survival rate, with 705 cases in the HR group and 826 cases in the RFA group. The heterogeneity test (I2=45%, P=0.08) showed no heterogeneity, so a fixed-effects model was used for meta-analysis. The meta-analysis results showed that the postoperative 1-year overall survival rate of the RFA group was better than that in the HR group in the treatment of single small HCC, and the difference was statistically significant (OR =1.57, 95% CI: 1.14, 2.17, P<0.05), as shown in Figure 2.
Postoperative 3-year overall survival rate
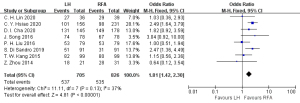
Eight articles (18-21,24-26,28) reported on the postoperative 3-year overall survival rate, including 705 cases in the HR group and 826 cases in the RFA group. The heterogeneity test (I2=37%, P=0.13) showed that there was no heterogeneity, so a fixed-effects model was used for meta-analysis. The meta-analysis results showed that the postoperative 3-year overall survival rate of the RFA group was better than that in the HR group in the treatment of single small HCC, and the difference was statistically significant (OR =1.81, 95% CI: 1.42, 2.30, P<0.05), as shown in Figure 3.
Postoperative 5-year overall survival rate
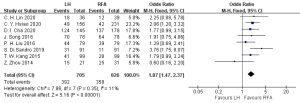
Eight articles (18-21,24-26,28) reported on the postoperative 5-year overall survival rate, including 705 cases in the HR group and 826 cases in the RFA group. The heterogeneity test (I2=11%, P=0.35) showed that there was no heterogeneity, so a fixed-effects model was adopted for meta-analysis. The meta-analysis results showed that postoperative 5-year overall survival rate of the RFA group was better than that in the HR group in the treatment of single small HCC, and the difference was statistically significant (OR =1.87, 95% CI: 1.47, 2.37, P<0.05), as shown in Figure 4.
Complications
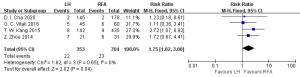
Postoperative complications were reported in four studies (18,21,27,28), including 704 cases in the RFA group and 353 cases in the HR group. The heterogeneity test (I2=0%, P=0.65) showed no heterogeneity, and so a fixed-effects model was used for meta-analysis. The meta-analysis results showed that the RFA group had fewer complications in the treatment of single small HCC than the HR group, and the difference was statistically significant [risk ratio (RR) =1.75, 95% CI: 1.02, 3.00, P<0.05], as shown in Figure 5.
Length of hospital stay
Five studies (21,23,26-28) reported on the length of hospital stay, with 640 patients in the RFA group and 314 in the HR group. The heterogeneity test (I2=99%, P<0.1) showed that there was heterogeneity, so a random-effects model was employed for meta-analysis. The meta-analysis results indicated that the length of hospital stay in the RFA group in the treatment of single small HCC was shorter than that of the HR group, and the difference was statistically significant (SMD =2.92, 95% CI: 0.54, 5.30, P<0.05), as shown in Figure 6.
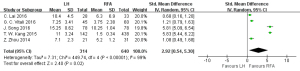
Recurrence
Six articles (19,20,22,23,25,26) reported on single small HCC recurrence, with 544 cases in the RFA group and 464 cases in the HR group. Since the heterogeneity test (I2=47%, P=0.11) showed no heterogeneity, a fixed-effects model was used for meta-analysis. The meta-analysis results demonstrated that the recurrence rate of single small HCC treated with RFA was higher than that in the HR group, and the difference was statistically significant (OR =0.49, 95% CI: 0.36, 0.66, P<0.05), as shown in Figure 7.
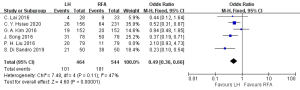
Operation time
Four articles (23,26-28) reported the operation time, involving 202 cases in the RFA group and 172 cases in the HR group. The heterogeneity test (I2=44%, P=0.15) showed no heterogeneity, and a fixed-effects model was used for meta-analysis. The meta-analysis results indicated that the operation time of the RFA group in the treatment of single small HCC was less than that of the HR group, and the difference was statistically significant (SMD =2.87, 95% CI: 2.57, 3.16, P<0.05), as shown in Figure 8.
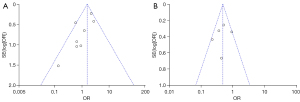
Publication bias and sensitivity analysis
RevMan 5.4 software was used to draw an inverted funnel plot to evaluate the risk of publication bias. The results showed that the inverted funnel plot was not bilaterally symmetrical, suggesting a high possibility of publication bias in the included studies (Figure 9A,9B). The forest plot of the meta-analysis showed the observed indicator of hospital stay was highly heterogeneous, and the sequential removal of articles did not have a significant impact on the results. This indicated that the bias caused by a single study was small, and the results of this meta-analysis were relatively stable. The majority of patients treated with RFA for single small HCC had a shorter operation time, which did not affect the overall judgment.
Discussion
Liver cancer has a hidden onset, rapid progression, and high mortality (29). As a special subtype of liver cancer, single small HCC is relatively common and is characterized by solitary onset with a diameter <5 cm, although there is currently no unified diagnostic standard (30). At present, surgical resection is one of the most effective treatments for single small HCC. With the continuous development of laparoscopic surgical techniques, laparoscopic hepatectomy has been increasingly widely used in clinical practice (31). Hepatectomy can completely remove the cancerous liver tissue and surrounding tissues. With the development of surgical techniques, the safety of surgical treatment has also been greatly improved, but still has some disadvantages, including trauma, long operation time, and excessive bleeding. Also, the mortality rate for surgery ranges from 1.6% to 10% (29,32).
Over the past decade, with the emergence and continuous improvement of new medical technologies and the popularity of minimally invasive and precise treatment concepts, RFA technology has achieved rapid development and is gradually being favored by clinicians. It has been widely used in clinical practice, especially in the treatment of single small HCC (33). RFA is a thermal ablation technology, which can generate high-speed ion vibration in the liver parenchyma through electroacupuncture with high-frequency current, and generate heat energy by violent intermolecular collision and friction. This induces irreversible changes in tumor cell membranes, increased cell membrane permeability, and disorder of the intracellular environment. Meanwhile, high temperature affects the deoxyribonucleic acid (DNA) metabolism of cells and tumor cell reproduction, leading to coagulation necrosis of cancer tumors and surrounding blood vessels, thereby destroying the tumor lesions (34).
RFA is currently the most widely used ablation technique and has been proven to be a safe and reliable technique. Especially under the guidance of ultrasound, the range of RFA is more precise, and the damage to the surrounding normal liver tissue is minimized. RFA is more effective in the treatment of severe liver cirrhosis, reduced liver function, and single small HCC in specific parts (35-37). Hasegawa et al. (38) reported that although RFA is safer, with fewer complications and a shorter operation time, it has a higher tumor recurrence rate than HR. This is consistent with the results obtained in the present meta-analysis, which may be due to the fact that ablation therapy only focuses on eliminating existing lesions, while surgical resection will remove a certain range of normal liver tissue at the tumor site, thereby avoiding residual recurrence of tumor tissue to the greatest extent. The high recurrence rate of RFA will affect the confidence of patients seeking medical treatment to a certain extent, and also reflects the risk of HCC as well as the insufficiency of currently available treatment methods. Thus, it is still necessary to explore new and effective methods for the treatment of HCC (39).
The results of this meta-analysis showed that single small HCC relapse was more likely to occur after RFA treatment, which may be attributable to the fact that the range of RFA is usually 3–5 cm. If the tumor diameter is greater than 5 cm, multiple ablations are required to eliminate the lesion, and each damaged area may leave a blind spot, thereby leading to incomplete tumor ablation and increased likelihood of local recurrence (40). Primary HCC is prone to recurrence, with more than 80% of cases recurring in the liver. The recurrence of HCC is multi-center growth, with many hidden cancer foci. On the premise of ensuring the patient’s own needs, liver segment resection and sub-segment resection are generally applied in surgery. At present, RFA cannot completely remove the small and hidden lesions, so the recurrence rate is higher than that of HR (41). However, even precise HR for the treatment of single small HCC still has a high long-term recurrence rate and poor prognosis (42). Song et al. (43) compared the short- and long-term results of laparoscopic hepatectomy and percutaneous RFA for a single HCC smaller than 4 cm in diameter. The complication rate of laparoscopic hepatectomy was significantly higher (28.2%, P=0.004) and the length of hospital stay was longer (15 days, P<0.0001), which was basically consistent with the results of the present study.
RFA has the characteristics of low trauma, few postoperative complications, short hospital stay, and reproducibility, and is more suitable than HR for recurrent small HCCs that meet the Milan criteria. An increasing number of patients are actively accepting this treatment method. Nevertheless, this study still has some limitations that should be noted. Firstly, no randomized controlled studies were included. Also, the diameter and number of tumors of the included patients varied, and the number of RFA implementations was not reported. Therefore, RFA cannot be regarded as the first choice for the treatment of HCCs meeting the Milan criteria. Therefore, multi-center, high-quality, prospective, randomized controlled trials are still needed for further validation.
Conclusions
The clinical efficacy of RFA in the treatment of single small HCCs meeting the Milan criteria is better than that of HR, and has the advantages of being minimally invasive, repeatability, fewer complications, and shorter operation time. Therefore, RFA can be given priority in the treatment of single small HCC.
Acknowledgments
Funding: This work was supported by the Chongqing Science and Health Joint Medical Research Project (No. 2020FYYX248) and Kuanren Leading Talent Project (No. kryc-lj-2108).
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-563/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-563/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zou Y, Guo CG, Zhang MM. Inhibition of human hepatocellular carcinoma tumor angiogenesis by siRNA silencing of VEGF via hepatic artery perfusion. Eur Rev Med Pharmacol Sci 2015;19:4751-61. [PubMed]
- Maucort-Boulch D, de Martel C, Franceschi S, et al. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer 2018;142:2471-7. [Crossref] [PubMed]
- Conroy MR, Moe TG. Hepatocellular carcinoma in the adult Fontan patient. Cardiol Young 2017;27:407-9. [Crossref] [PubMed]
- Hokuto D, Nomi T, Yamato I, et al. The prognosis of liver resection for patients with four or more colorectal liver metastases has not improved in the era of modern chemotherapy. J Surg Oncol 2016;114:959-65. [Crossref] [PubMed]
- Kim JM. Can hepatocellular carcinoma recurrence be prevented after liver transplantation? Clin Mol Hepatol 2021;27:562-3. [Crossref] [PubMed]
- Clark T, Maximin S, Meier J, et al. Hepatocellular Carcinoma: Review of Epidemiology, Screening, Imaging Diagnosis, Response Assessment, and Treatment. Curr Probl Diagn Radiol 2015;44:479-86. [Crossref] [PubMed]
- Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol 2017;34:153-9. [Crossref] [PubMed]
- Fiorentino A, Alongi F. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Hepatocellular Carcinoma: No Way Out Without a Randomized Trial? J Clin Oncol 2018;36:2558-9. [Crossref] [PubMed]
- Zhu L, Hao X, Wang L, et al. Clinical efficacy of radiofrequency ablation combined with splenectomy for small hepatocellular carcinoma with hypersplenism. Panminerva Med 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Sartori S, Tombesi P, Di Vece F. Thermal ablation in colorectal liver metastases: Lack of evidence or lack of capability to prove the evidence? World J Gastroenterol 2016;22:3511-5. [Crossref] [PubMed]
- Vogl TJ, Farshid P, Naguib NN, et al. Thermal ablation of liver metastases from colorectal cancer: radiofrequency, microwave and laser ablation therapies. Radiol Med 2014;119:451-61. [Crossref] [PubMed]
- Vogl TJ, Zegelman A, Bechstein WO, et al. Treatment of liver metastases of colorectal carcinoma: overview of hyperthermal ablation methods. Dtsch Med Wochenschr 2013;138:792-8. [PubMed]
- Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol 1996;167:759-68. [Crossref] [PubMed]
- Sotiropoulos GC, Lang H, Frilling A, et al. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology 2006;53:322-9. [PubMed]
- Feng X, Pai M, Mizandari M, et al. Towards the optimization of management of hepatocellular carcinoma. Front Med 2011;5:271-6. [Crossref] [PubMed]
- Huang J, Hernandez-Alejandro R, Croome KP, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma in Childs A cirrhotics-a retrospective study of 1,061 cases. J Gastrointest Surg 2011;15:311-20. [Crossref] [PubMed]
- Vivarelli M, Guglielmi A, Ruzzenente A, et al. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg 2004;240:102-7. [Crossref] [PubMed]
- Cha DI, Song KD, Kang TW, et al. Small masses (≤3 cm) diagnosed as hepatocellular carcinoma on pre-treatment imaging: comparison of therapeutic outcomes between hepatic resection and radiofrequency ablation. Br J Radiol 2020;93:20190719. [Crossref] [PubMed]
- Di Sandro S, Benuzzi L, Lauterio A, et al. Single Hepatocellular Carcinoma approached by curative-intent treatment: A propensity score analysis comparing radiofrequency ablation and liver resection. Eur J Surg Oncol 2019;45:1691-9. [Crossref] [PubMed]
- Hsiao CY, Hu RH, Ho CM, et al. Surgical resection versus radiofrequency ablation for Barcelona Clinic Liver Cancer very early stage hepatocellular carcinoma: long-term results of a single-center study. Am J Surg 2020;220:958-64. [Crossref] [PubMed]
- Kang TW, Kim JM, Rhim H, et al. Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection--Propensity Score Analyses of Long-term Outcomes. Radiology 2015;275:908-19. [Crossref] [PubMed]
- Kim GA, Shim JH, Kim MJ, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg 2016;103:126-35. [Crossref] [PubMed]
- Lai C, Jin RA, Liang X, et al. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B 2016;17:236-46. [Crossref] [PubMed]
- Lin CH, Ho CM, Wu CH, et al. Minimally invasive surgery versus radiofrequency ablation for single subcapsular hepatocellular carcinoma ≤ 2 cm with compensated liver cirrhosis. Surg Endosc 2020;34:5566-73. [Crossref] [PubMed]
- Liu PH, Hsu CY, Hsia CY, et al. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma ≤ 2 cm in a Propensity Score Model. Ann Surg 2016;263:538-45. [Crossref] [PubMed]
- Song J, Wang Y, Ma K, et al. Laparoscopic hepatectomy versus radiofrequency ablation for minimally invasive treatment of single, small hepatocellular carcinomas. Surg Endosc 2016;30:4249-57. [Crossref] [PubMed]
- Vitali GC, Laurent A, Terraz S, et al. Minimally invasive surgery versus percutaneous radio frequency ablation for the treatment of single small (≤3 cm) hepatocellular carcinoma: a case-control study. Surg Endosc 2016;30:2301-7. [Crossref] [PubMed]
- Zhou Z, Lei J, Li B, et al. Liver resection and radiofrequency ablation of very early hepatocellular carcinoma cases (single nodule <2 cm): a single-center study. Eur J Gastroenterol Hepatol 2014;26:339-44. [Crossref] [PubMed]
- Song KD, Lim HK, Rhim H, et al. Repeated Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Hepatic Resection: A Propensity Score Matching Study. Radiology 2015;275:599-608. [Crossref] [PubMed]
- Zhou Y, Shen DH, Gao ZF, et al. Primary hepatocellular carcinoma with small lymphocytic lymphoma: report of a case. Zhonghua Bing Li Xue Za Zhi 2020;49:373-5. [PubMed]
- De Mattia E, Cecchin E, Guardascione M, et al. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J Gastroenterol 2019;25:3870-96. [Crossref] [PubMed]
- Choti MA. Surgical management of hepatocellular carcinoma: resection and ablation. J Vasc Interv Radiol 2002;13:S197-203. [Crossref] [PubMed]
- Yu HC, Cheng JS, Lai KH, et al. Factors for early tumor recurrence of single small hepatocellular carcinoma after percutaneous radiofrequency ablation therapy. World J Gastroenterol 2005;11:1439-44. [Crossref] [PubMed]
- Yu H. Study on the propensity score matching of radiofrequency ablation and laparoscopic hepatectomy for single liver cancer with diameter less than 5 cm. Dissertation. Nanchang: Nanchang University, 2021.
- Izumi N. Recent advances of radiofrequency ablation for early hepatocellular carcinoma. J Gastroenterol Hepatol 2011;26:115-22. [Crossref] [PubMed]
- Kim YS, Lim HK, Rhim H, et al. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2014;28:897-908. [Crossref] [PubMed]
- Zhu F, Rhim H. Thermal ablation for hepatocellular carcinoma: what's new in 2019. Chin Clin Oncol 2019;8:58. [Crossref] [PubMed]
- Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 2013;58:724-9. [Crossref] [PubMed]
- Sakon M, Ariyoshi H, Umeshita K, et al. Ischemia-reperfusion injury of the liver with special reference to calcium-dependent mechanisms. Surg Today 2002;32:1-12. [Crossref] [PubMed]
- Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10-24. [Crossref] [PubMed]
- Cho JY, Han HS, Choi Y, et al. Association of Remnant Liver Ischemia With Early Recurrence and Poor Survival After Liver Resection in Patients With Hepatocellular Carcinoma. JAMA Surg 2017;152:386-92. [Crossref] [PubMed]
- Hibi T, Sakamoto Y, Asamura H, et al. Successful resection of hepatocellular carcinoma with bronchobiliary fistula caused by repeated transcatheter arterial embolizations: Report of a case. Surg Today 2007;37:154-8. [Crossref] [PubMed]
- Song I, Rhim H, Lim HK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol 2009;19:2630-40. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



