
Effects of long-term exposure to sevoflurane on the proliferation, migration, invasion, and cisplatin sensitivity of esophageal cancer
Introduction
Esophageal carcinoma ranks as the sixth highest cause of male cancer-related mortality and eighth most common cancer worldwide (1). Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EADC) are the two most common histological subtypes (2). EADC is more prevalent in Western countries, while East Asian countries have the highest incidence of ESCC (3). The overall 5-year survival rate for esophageal cancer is approximately 15–25% worldwide, and improving survival rates for this disease is a critical issue.
Cancer stem cells (CSCs) are tumor cells with the potential for self-renewal and multi-differentiation and play a leading role in tumor initiation, metastasis, immune evasion, and resistance to existing treatment methods. Octamer-binding transcription factor 4 (OCT4) and (sex determining region Y)-box 2 (SOX2) are representative embryonic stem cell transcription factors. Both of these proteins play a decisive role in the process of embryonic stem cell maintenance and multi-directional development. Several studies have shown that SOX2 (4,5) and OCT4 (6,7) play an important role in the resistance of tumor cells to chemotherapy, radiotherapy, and targeted therapies. Relevant studies have shown that one of the main reasons for the failure of esophageal cancer therapy is the emergence of drug-resistant cancer cells after treatment and subsequent recurrence of the cancer (8).
Surgery, comprehensive treatment, radiotherapy, and chemotherapy are the main treatment methods for esophageal cancer (9-11). Certain factors during the perioperative period can affect recurrence and the metastatic potential of cancer, including the surgical procedure itself (12), anesthetics (13,14), pain (15), and the stress response to the surgery (16). In recent years, it has been reported that anesthesia may have a direct effect on tumor cells and may affect tumor recurrence, metastasis, and long-term survival. Sevoflurane is one of the most commonly used clinical inhalation anesthetics, and its impact on tumors is controversial (17). Studies have shown that sevoflurane inhibited the proliferation, metastasis, and recurrence of various tumors after surgery, including breast cancer (18), ovarian cancer (19), and colon cancer (20). However, other studies reported opposite results (21-23).
There are few studies on the effects of sevoflurane on postoperative metastasis and recurrence of esophageal cancer. At present, there are no reports on the effects of sevoflurane on postoperative tumor stem cell expression and sensitivity to chemotherapy in patients with esophageal cancer. Therefore, this study aimed to determine the effects of long-term exposure to sevoflurane on the proliferation, migration, and invasion ability of esophageal cancer cells. Furthermore, tumor stemness and its potential impact on the sensitivity of cancer cells to chemotherapy was explored. This study was conducted to provide a theoretical basis for the use of anesthetics to reduce the metastasis and recurrence of esophageal cancer and promote the curative effects of anti-cancer treatments. We present the protocol in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2404/rc).
Methods
Cell culture
Two esophageal cancer cell lines EC109 and SKGT-4 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). EC109 and SKGT-4 are highly differentiated ESCC and EADC lines, respectively. The two cell lines were cultured in RPMI 1640 (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Inc.) and 100 μg/mL streptomycin and 100 Units/mL penicillin G (Sigma‑Aldrich; Merck KGaA, Darmstadt, Germany). The cells were subcultured every 48 h and maintained at 37 °C and 5% CO2 in a humidified incubator.
Sevoflurane exposure and combined treatment
Cells from the sevoflurane group were placed in a small sealed chamber that was connected to an anesthetic vaporizer (Dräger, Lübeck, Germany) and incubated for 10 h. The vaporizer transported gases into the chamber that consisted of 5% CO2, 21% O2, and 74% N2. Prior to beginning the experiment, the vaporizer delivered sevoflurane into the chamber at a rate of 2 L per min until the gas in the chamber was in a stable state. Sevoflurane concentrations in the chamber were analyzed using a gas monitor (PM 8060, Dräger) until the sevoflurane concentration reached 3%. Cells from the control group were placed in a sealed chamber under the same conditions except for the presence of sevoflurane gas. Cells from the cisplatin group were treated with 10 μmol/L cisplatin. Cells from the sevoflurane plus cisplatin group were treated with 10 μmol/L cisplatin and immediately exposed to 3% sevoflurane for 10 h.
MTT assay
The methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay was used to determine cell proliferation and viability. MTT was dissolved in serum-free RPMI culture medium. MTT was added at a final concentration of 0.5 mg/mL to the cells and incubated for 4 h. After washing the cells with 1 mL phosphate-buffered saline (PBS), 1 mL of dimethyl sulfoxide was added, and the cells were dissolved completely by gentle rocking for 20 min. The absorbance per well was measured at 570 nm using a microplate reader.
Scratch assay
The scratch assay is a convenient method for analysis of cell migration in vitro. Cells were cultured in 6-well plates at 37 °C, 5% CO2. When the cell density was greater than 90%, one artificial gap per well was created by scratching the cells with a 200 μL micropipette tip. Images were collected and analyzed at different time points.
Transwell assays
The effects of sevoflurane on cell invasive capability were detected using 24-well transwell plates (8 µm pore size; Corning, NY, USA). Briefly, the bottom of each well insert was coated with 200 mg/mL of Matrigel and allowed to dry. Cells (5×104) in 200 μL serum-free medium were added to each upper chamber (insert). RPMI 1640 medium supplemented with 20% fetal bovine serum was added to the lower chambers. Following incubation for 48 h at 37 °C, invasive cells in the lower chamber were fixed with paraformaldehyde, colored with crystal violet, and counted using an inverted microscope (Olympus, Tokyo, Japan).
Sphere-forming assay
The sphere-forming assay can effectively detect the capacity of CSCs to self-renew. Cells (1×103) were cultured in serum-free medium supplemented with heparin, B27, epidermal growth factor, and basic fibroblast growth factor in 24-well ultra-low attachment plates (Corning, NY, USA) for one week, and the spheres were counted using an inverted microscope.
Western blot
Cells were lysed for 30 min on ice in RIPA lysis buffer (Solarbio, Beijing, China) containing phenylmethylsulfonyl fluoride (1,000:1) and centrifuged to collect the supernatants. The protein concentration in each supernatant was determined using the BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). Protein lysates were incubated in a 95 °C water bath for 10 min in Lithium Dodecyl Sulfate (LDS) sample buffer (4×), and proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Solarbio, Beijing, China). After transfer, the membranes were blocked with 7% skim milk for 60 min at room temperature and incubated overnight at 4 °C with the following primary antibodies: anti-SOX2 (1:1,000, Abcam, Cambridge, MA, USA), anti-OCT4 (1:1,000, Abcam), and anti-β-actin (1:1,000, Abcam). The membranes were washed and incubated with secondary antibodies for 90 min at room temperature. Proteins were visualized using the ECL Plus Kit (Biyuntian, China) and analyzed using image analysis software (ImageJ, version 1.48; National Institutes of Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
ELISA is a qualitative and quantitative method for the detection of immune responses using the specific binding of antigens and antibodies. Briefly, the culture medium in the experimental groups and control group were collected at indicated time and centrifuged at 2,500 rpm for 10 min. The levels of lactate dehydrogenase (LDH) in the supernatants were measured following the instructions from the enzyme immune assay (R&D Systems, Oxon, UK).
Analysis of cell cycle by flow cytometry
Cells were collected and washed with PBS by centrifugation at 2,500 rpm for 10 min. The cell concentration was adjusted to 1×106/mL, and the cells were fixed with 500 μL 70% pre-cooled ethanol overnight. The cells were washed with PBS, centrifuged, and the supernatant was removed. RNase A solution and propidium iodide (PI; both from Solarbio, Beijing, China) were added to the cells and incubated at 4 °C for 30 min in the dark. The cell cycle distribution was analyzed by flow cytometry.
Detection of apoptosis by flow cytometry
The adherent cells were detached from the cell culture flask with 0.25% trypsin without ethylenediaminetetraacetic acid (PYG0107, Boster, Wuhan, Hubei, China) and collected for staining with PI and fluorescein isothiocyanate (FITC)-conjugated Annexin-V following the manufacturer’s instructions from the Annexin-V FLUOS staining kit (Solarbio, Beijing, China). Apoptotic cells were detected by flow cytometry following the manufacturer’s protocol.
Statistical analysis
All statistical analysis was performed using GraphPad Prism (GraphPad Software, CA, USA). All data were presented as mean ± SEM. Significance was determined by Student’s t-test or one-way analysis of variance (ANOVA) followed by Tukey’s test. P<0.05 was considered statistically significant.
Results
Effect of sevoflurane on the proliferation, migration, and invasion of EC109 and SKGT-4 cells
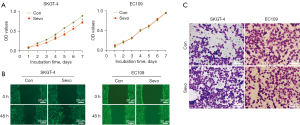
To evaluate the effect of sevoflurane on the biological properties of EC109 and SKGT-4 cells, we performed proliferation, migration, and invasion assays. The data showed that exposure to sevoflurane significantly inhibited the proliferation (Figure 1A) and promoted the migration (Figure 1B) and invasion (Figure 1C) of SKGT-4 cells when compared with the control group. However, sevoflurane did not affect these properties in EC109 cells.
Effect of sevoflurane on cell cycle and cell stemness in SKGT-4 cells
To investigate whether sevoflurane-mediated inhibition of cell proliferation was caused by cell cycle arrest, we analyzed cell cycle distribution in EC109 and SKGT-4 cells using flow cytometry. In EC109 cells, exposure to sevoflurane did not affect the cell cycle; however, in SKGT-4 cells exposed to sevoflurane, there was a significant increase in the number of cells in S phase and decrease in the number of cells in G2/M phase compared with the control group. There was no difference in the number of SKGT-4 cells in the Go/G1 phase between the sevoflurane and control groups (Figure 2A).
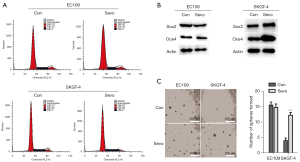
The presence of CSCs is closely related to the metastasis of tumor cells. To investigate whether sevoflurane-mediated migration and invasion of SKGT-4 cells were related to CSCs, we performed western blots and sphere-forming assays. Western blots showed that the protein levels of OCT4 and SOX2 in SKGT-4 cells were significantly increased compared with the control group (Figure 2B), suggesting that sevoflurane increased the stemness potential of SKGT-4 cells. The sphere-forming assay indicated that the stem cell-like properties of SKGT-4 cells were significantly enhanced after exposure to sevoflurane compared with the control group (Figure 2C).
Effect of sevoflurane on sensitivity to cisplatin
Studies have shown that CSCs negatively affect the efficacy of anticancer chemotherapy. To explore the effects of sevoflurane on cisplatin resistance in esophageal cancer cells, the LDH content and apoptosis were measured in the two cell lines. The LDH content (Figure 3A) and the number of cells undergoing apoptosis (Figure 3B) were significantly reduced in the SKGT-4 cells treated with sevoflurane plus cisplatin compared with cisplatin alone. For the EC109 cells, there were no differences in LDH levels or apoptosis between cells treated with cisplatin and sevoflurane plus cisplatin. These data suggested that sevoflurane exposure reduced cisplatin sensitivity of the EADC cells SKGT-4 and the curative effect of chemotherapy.
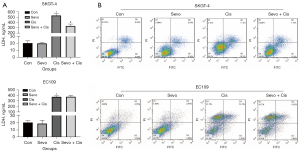
Discussion
Surgery is one of the main treatments for patients with esophageal cancer (9,11). Nevertheless, postoperative recurrence is frequent. Multiple factors may contribute to postoperative recurrence and metastasis, including the anesthetics used (24). Sevoflurane is widely used in general anesthesia; however, the role of sevoflurane in esophageal cancer recurrence is unknown. In this study, we reported for the first time that long-term exposure to sevoflurane inhibited the proliferation and promoted the migration and invasion of SKGT-4 cells.
Cell proliferation can be used as an important indicator of cell survival or death (25). Tumor metastasis is a complex process involving the spread of tumor cells from the primary site to distant organs and plays a vital role in the development of drug resistance (26). The role of sevoflurane in postoperative recurrence and metastasis of cancer is controversial (17,27). Shi et al. showed that sevoflurane promoted the self-renewal and proliferation of CSCs through hypoxia-inducible factors, which may promote tumor growth (24). However, another study reported that sevoflurane anesthesia induced apoptosis and autophagy and inhibited the proliferation and invasion of colonic tumor cells (19). In this study, we concluded that long-term exposure to sevoflurane inhibited the proliferation of SKGT-4 cells, while promoting the migration and invasion capacity of these cells.
CSCs have stem cell-like characteristics and play a key role in postoperative recurrence and metastasis (28). SOX2 and OCT4 regulate the self-renewal of CSCs and multi-directional development of embryonic stem cells (4,7). In our study, long-term exposure to sevoflurane increased the stemness of SKGT-4 cells and significantly increased the protein expression of SOX2 and OCT4. Studies have shown that SOX2 and OCT4 can promote the progression of tumor cells (21,29). Thus, sevoflurane may have promoted the metastatic and recurrence potential of SKGT-4 cells by increasing the expression of SOX2 and OCT4 and enhancing stemness. However, sevoflurane did not affect EC109 cells. We believe that the different effects of sevoflurane on these two esophageal cancer cell lines may be related to the concentration and time of sevoflurane treatment and the histological and oncological differences between these tumor subtypes.
The development of chemoresistance is common during the treatment of patients with esophageal cancer. Therefore, it is necessary to find new therapeutic strategies. Cisplatin is a commonly used chemotherapy drug for patients with esophageal cancer and resistance to cisplatin substantially reduces the efficacy of the chemotherapeutic regimen. Several studies have reported the effect of sevoflurane on the sensitivity of different types of cancer cells to cisplatin, and the results varied (17,30,31). In our study, the combined use of sevoflurane and cisplatin inhibited the apoptosis of SKGT-4 cells compared with cisplatin alone. SOX2 and OCT4 play an important role in the resistance of tumor cells to chemotherapy, radiotherapy, and targeted therapy (4-7). Thus, we propose that long-term exposure to sevoflurane increased the protein expression of SOX2 and OCT4 that reduced the sensitivity of SKGT-4 cells to cisplatin, and this mechanism may ultimately promote postoperative recurrence and metastasis.
There were limitations in this study. First, further research will be required to explore the potential molecular mechanism or signal transduction pathways by which sevoflurane may produce cisplatin-resistance in EADC. Second, animal experiments were not performed, which limits the translation of these in vitro experiments to clinical applications. In summary, our results suggest that long-term exposure to sevoflurane may promote the migration and invasion and increase the stemness of EADC cells, thereby reducing chemosensitivity to cisplatin and ultimately promoting metastasis and recurrence of EADC.
Conclusions
Based on our findings, it is proposed that long-term exposure to sevoflurane should be cautious during the perioperative period to decrease the metastasis and recurrence of esophageal cancer. Our findings provide a theoretical basis for the use of anesthetics to reduce the metastasis and recurrence of esophageal cancer and promote the curative effects of anti-cancer treatments.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2404/rc
Data Sharing Statement: The authors have completed the Data sharing Statement form. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2404/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2404/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Hüser L, Novak D, Umansky V, et al. Targeting SOX2 in anticancer therapy. Expert Opin Ther Targets 2018;22:983-91. [Crossref] [PubMed]
- Novak D, Hüser L, Elton JJ, et al. SOX2 in development and cancer biology. Semin Cancer Biol 2020;67:74-82. [Crossref] [PubMed]
- Villodre ES, Kipper FC, Pereira MB, et al. Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat Rev 2016;51:1-9. [Crossref] [PubMed]
- Mohiuddin IS, Wei SJ, Kang MH. Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim Biophys Acta Mol Basis Dis 2020;1866:165432. [Crossref] [PubMed]
- Yue D, Zhang Z, Li J, et al. Transforming growth factor-beta1 promotes the migration and invasion of sphere-forming stem-like cell subpopulations in esophageal cancer. Exp Cell Res 2015;336:141-9. [Crossref] [PubMed]
- Deng HY, Wang WP, Wang YC, et al. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg 2017;51:421-31. [PubMed]
- Mönig S, Chevallay M, Niclauss N, et al. Early esophageal cancer: the significance of surgery, endoscopy, and chemoradiation. Ann N Y Acad Sci 2018;1434:115-23. [Crossref] [PubMed]
- Leng XF, Daiko H, Han YT, et al. Optimal preoperative neoadjuvant therapy for resectable locally advanced esophageal squamous cell carcinoma. Ann N Y Acad Sci 2020;1482:213-24. [Crossref] [PubMed]
- Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med 2018;16:8. [Crossref] [PubMed]
- Lai HC, Lee MS, Lin C, et al. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma: a retrospective cohort study. Br J Anaesth 2019;123:151-60. [Crossref] [PubMed]
- Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 2019;394:1807-15. [Crossref] [PubMed]
- Li J, Sun Y, Ding G, et al. Persistent pain accelerates xenograft tumor growth of breast cancer in rat. Biochem Biophys Res Commun 2018;495:2432-8. [Crossref] [PubMed]
- Chen Z, Zhang P, Xu Y, et al. Surgical stress and cancer progression: the twisted tango. Mol Cancer 2019;18:132. [Crossref] [PubMed]
- Ciechanowicz S, Zhao H, Chen Q, et al. Differential effects of sevoflurane on the metastatic potential and chemosensitivity of non-small-cell lung adenocarcinoma and renal cell carcinoma in vitro. Br J Anaesth 2018;120:368-75. [Crossref] [PubMed]
- Liu J, Yang L, Guo X, et al. Sevoflurane suppresses proliferation by upregulating microRNA-203 in breast cancer cells. Mol Med Rep 2018;18:455-60. [Crossref] [PubMed]
- Zhang C, Wang B, Wang X, et al. Sevoflurane inhibits the progression of ovarian cancer through down-regulating stanniocalcin 1 (STC1). Cancer Cell Int 2019;19:339. [Crossref] [PubMed]
- Yang X, Zheng YT, Rong W. Sevoflurane induces apoptosis and inhibits the growth and motility of colon cancer in vitro and in vivo via inactivating Ras/Raf/MEK/ERK signaling. Life Sci 2019;239:116916. [Crossref] [PubMed]
- Jen J, Tang YA, Lu YH, et al. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer 2017;16:104. [Crossref] [PubMed]
- Xue F, Xu Y, Song Y, et al. The Effects Of Sevoflurane On The Progression And Cisplatinum Sensitivity Of Cervical Cancer Cells. Drug Des Devel Ther 2019;13:3919-28. [Crossref] [PubMed]
- Li R, Huang Y, Lin J. Distinct effects of general anesthetics on lung metastasis mediated by IL-6/JAK/STAT3 pathway in mouse models. Nat Commun 2020;11:642. [Crossref] [PubMed]
- Shi QY, Zhang SJ, Liu L, et al. Sevoflurane promotes the expansion of glioma stem cells through activation of hypoxia-inducible factors in vitro. Br J Anaesth 2015;114:825-30. [Crossref] [PubMed]
- Adan A, Kiraz Y, Baran Y. Cell Proliferation and Cytotoxicity Assays. Curr Pharm Biotechnol 2016;17:1213-21. [Crossref] [PubMed]
- Singh M, Yelle N, Venugopal C, et al. EMT: Mechanisms and therapeutic implications. Pharmacol Ther 2018;182:80-94. [Crossref] [PubMed]
- Zhang W, Sheng B, Chen S, et al. Sevoflurane Enhances Proliferation, Metastatic Potential of Cervical Cancer Cells via the Histone Deacetylase 6 Modulation In Vitro. Anesthesiology 2020;132:1469-81. [Crossref] [PubMed]
- Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol 2019;234:8381-95. [Crossref] [PubMed]
- Maurizi G, Verma N, Gadi A, et al. Sox2 is required for tumor development and cancer cell proliferation in osteosarcoma. Oncogene 2018;37:4626-32. [Crossref] [PubMed]
- Sugimoto H, Kawaraguchi Y, Nomura Y, et al. Exposure to 1% Sevoflurane for 6 Hours Enhances Proliferation of Human Colon Cancer Cells. Masui 2015;64:357-61. [PubMed]
- Nishiwada T, Kawaraguchi Y, Uemura K, et al. Effect of sevoflurane on human hepatocellular carcinoma HepG2 cells under conditions of high glucose and insulin. J Anesth 2015;29:805-8. [Crossref] [PubMed]


