
Primary pulmonary choriocarcinoma in male: report a case with genetic testing and review of the literature
Introduction
Choriocarcinoma is a highly malignant trophoblastic tumor that secretes β-human chorionic gonadotropin (HCG), often occurring in intrauterine pregnancy. It is scarce to occur outside the gonads. Primary pulmonary choriocarcinoma (PPC) is exceedingly rare, especially in males (1). By reporting a case of male PPC, we hope to improve the accuracy of the diagnosis and provide new insights into the pathogenesis and treatment of the disease by analyzing previous literature and genetic testing results. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2627/rc).
Case presentation
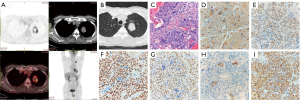
We reported that a 65-year-old man found solid nodules shadows in the lungs when undergoing the X-ray examination. After two weeks of anti-inflammatory treatment, the shadow was no significant change. After admission to our hospital, the routine physical examinations of this patient are normal, with testicles of equal size no nodules or lumps. Chest computed tomography (CT) revealed a 34 mm × 25 mm tumor in the upper left lung. After enhancement, there was no obvious enhancement, without pleura thickening and lymph node enlargement, and no pleural effusion. Routine laboratory tests were normal, and the HCG levels in the patient’s urine and blood were not tested before the operation. Preoperative PET ruled out metastatic disease and determined patient tolerance to surgical treatment (Figure 1A,1B). We initially considered that the patient might be a patient with lung cancer. The patient was in good health before, and there is no such disease patient in the family. The patient underwent three-dimension uniportal thoracoscopic left upper lung resection and lymph node dissection. The operation went well, and the patient was discharged four days later. The mass dimensions are 4.2 cm × 3.8 cm × 2.9 cm, The color is gray-brown, gray-red, slightly tough, and the boundary is still clear. Microscopic pathological: malignant tumor necrosis. Immunohistochemical results: HCG(+), catalase-like-3 (CATA-3)(+), SMARCA4(+), cytokeratin 7 (CK7)(+), cytokeratin (CK)(+), portion of P40(+), NUT(−), transcription termination factor 1 (TTF-1)(−), vimentin (VIM)(−) (Figure 1C-1I). Malignant trophoblastic tumors (chorionic carcinoma) were considered in combination with pathological and immunohistochemical staining. No lymph node metastasis was found in the dissection’s sub-aortic lymph nodes, para-aortic lymph nodes, subcarinal lymph nodes, pulmonary hilar lymph nodes, and interlobar lymph nodes. Postoperative pathological staging: pT2bN0M0, stage IIa, R0 resection.
After the operation, the patient has genetically tested: missense mutation in exon 8 of tumor protein p53 (TP53) gene (NM_000546.5), c.818G>T (p.R273L), abundance: 56.50%. NRAS proto-oncogene (NRAS) gene (NM_002524.4) copy number amplification, chromosome position: 1, copy number: 4. The frameshift mutation in exon 18 of fibroblast growth factor receptor 1 (FGFR1) gene (NM_023110.2) c.2370_2371del (p.E792fs), the abundance is 36.73%. Programmed death-ligand 1 (PD-L1) (1%< tumor cell <50%) (Figure 2).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Choriocarcinoma is a highly malignant tumor of trophoblast cells, mainly in women. Most choriocarcinoma originated from gonads, such as the ovaries and testes. Extragonadal choriocarcinoma generally occurs in the body's midline, such as the mediastinum, retroperitoneum, pineal gland, and the middle of the cranium (2,3). Review the literature from 1953 to 2021, 34 literature, including 41 cases of male PPC, were reported. Therefore, PPC is extremely rare.
The literature reviewed 41 male PPC patients ranging in age from 4 months to 77 years, with an average age of 49.7 years. Analyzing the 32 patients with relevant clinical information, only 4 patients were asymptomatic at the time of diagnosis. The majority of patients (28 cases) had symptoms, which counts 87.5%. The most common symptoms were coughing (16 cases), hemoptysis (15 cases), chest pain (9 cases), and 4 patients had symptoms of dyspnea. Gynecomastia is a noteworthy sign of the disease (4 cases). Most tumors occurred on the right side (14 cases), and some tumors occurred in both lungs (5 cases). In addition, pulmonary choriocarcinoma is prone to metastasis and recurrence. Totally, 19 patients (59.4%) were found to have metastasis and recurrence, including 11 brain metastases. For PPC, simple pulmonary choriocarcinoma (26 cases) is the most common type of pathology, and a few are combined with adenocarcinoma, small cell lung cancer, and embryonal cell carcinoma.
Due to the extremely low occurrence, imaging diagnostics is inexperienced. Pulmonary choriocarcinoma is usually shown a solid nodules shadow in CT, which is easily confused by lung cancer. Such patients have no obvious symptoms in the early stages. When the male patient finds the lung’s shadow with signs of gynecomastia, the possibility of pulmonary choriocarcinoma should be highly suspected. In addition, the blood HCG level of these patients is much higher than usual. Therefore, abnormally elevated blood HCG in patients has important prompting significance for diagnosing choriocarcinoma. Simultaneously, blood levels of HCG are also of particular importance for monitoring treatment efficacy and recurrence.
The pathomorphology of choriocarcinoma presents the tumor cells are arranged in nests and solid sheets, with large areas of necrosis and obvious hemorrhage. It consists of mononuclear trophoblast cells (including cytotrophoblast cells and intermediate trophoblast cells) with medium clear cytoplasm and large multinucleated giant cells (syncytiotrophoblasts). The latter has obvious atypia, abundant cytoplasm, pink staining, or vacuoles. The number and volume of multinucleated giant cells in primary choriocarcinoma are generally less and smaller than in giant cell carcinoma, and HCG expression is not as markedly elevated as in primary choriocarcinoma. In addition, when diagnosis, care should also be taken to exclude the possible diagnosis of giant cell anaplastic and undifferentiated carcinoma. Therefore, immunohistochemical staining results have a significant role in making an accurate diagnosis. Previously, our hospital has reported 20 cases of immunohistochemistry diagnosis experience of choriocarcinoma. The researchers believed that GATA-3, CK7, p63, p40, and CD10 positive, and CK5/6, TTF-1, Napsin A negative have important prompting function for the diagnosis of choriocarcinoma (4). Huang’s research indicated that the expression of GATA-3 may be a tissue-specific marker for trophoblast differentiation, which is of great significance for the diagnosis of choriocarcinoma (5). Gasparri’s team reported that CK7, AE1/AE3, and CD10 could be positive in pulmonary choriocarcinoma, while TTF-1 and inhibin are negative (6). Pulmonary choriocarcinoma is rare in clinical practice, easily misdiagnosed, and easily confused with squamous cell carcinoma, giant cell carcinoma, adenocarcinoma, and malignant melanoma. Therefore, microscopy combined with immunohistochemical results is essential for diagnosing choriocarcinoma.
The prognosis of PPC is extremely poor. Reviewing the previous literature, only six patients survived more than 1 year. Grouping the patients in the literature, compared with untreated patients, treated patients have a more extended survival period (P=0.0051). Depending on the treatment regimen, patients, including surgery, had better survival than patients without surgery (P=0.027) (Figure 3). While there is currently no uniform treatment guideline for this disease, after reviewing previous cases, we believe that surgical treatment could be a major treatment option for PPC, and it is of great significance to improve the survival rate of patients. In addition, due to the high degree of malignancy, rapid growth, recurrence, and metastasis of PPC, postoperative chemotherapy and preventive craniocerebral radiotherapy are needed to improve the patient’s survival. Moreover, with the increasing development of targeted therapies and immunotherapies, the efficacy of this type of treatment is also worth exploring.

Second-generation sequencing revealed a missense mutation in exon 8 of the patient’s TP53 gene (Nm_000546.5). The original arginine at position 273 of the gene was mutated to leucine, which changed the function of the protein it encodes, and the mutation of the encoded protein may be associated with the poor prognosis non-small cell lung cancer (NSCLC) (7-9). TP53 is the most common mutated gene in human cancer. Loss of gene function mutations will cause the encoded P53 protein to lose its regulatory effect on cell growth, apoptosis, and DNA repair, which will lead to tumors. The gene function disappeared of TP53 in tumors mainly due to single-nucleotide missense mutations, which are widely distributed throughout the gene. Unfortunately, no approved targeted drugs target this gene mutation for tumor treatment (10,11).
NRAS is a member of the RAS oncogene family and is functionally altered in various cancers. NRAS can promote tumorigenesis by activating PI3K/AKT, MAPK/ERK, NF-κB, and other downstream pathways. Meanwhile, NRAS is involved in EGFR signaling, and the NRAS mutation keeps the gene continuously active, leading to cells proliferating and eventually cancerous. Furthermore, the mutation status of NRAS has a decision-making effect on targeted clinical therapy (12). Clinical studies have found that the expression of PD-L1 is relatively high in patients with NRAS mutations in melanoma, and this type of patient may benefit from immune-based therapy (13).
The protein encoded by the FGFR1 is a member of the FGFR family. The receptor can promote cell proliferation and invasion by recognizing ligands and activating downstream signaling pathways such as MAPK, PI3K/ATK, PLCγ, and JAK-STAT. It is worth mentioning that the pan-FGFR inhibitor erdafitinib has been approved in locally advanced or metastatic bladder cancer with abnormal FGFR gene (14).
With the increasing status of immunotherapy in cancer treatment, immune checkpoint inhibitors, such as programmed death receptor 1 (PD-1)/PD-L1 inhibitors, have become a novel drug for tumor treatment and has played an essential role in many kinds of tumors (15). Genetic testing revealed the presence of PD-L1 in the patient’s tumor. Can the combination of immunotherapy and traditional chemotherapy improve patient survival?
Conclusions
PPC is an extremely rare and highly malignant tumor. There is no agreed treatment guideline. Once the diagnosis is confirmed, the comprehensive treatment of surgical resection combined with chemotherapy and radiotherapy is of great significance to improve the prognosis of patients. In addition, genetic and molecular testing of this type of disease can provide new ideas for exploring its pathogenesis, early diagnosis, and targeted therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2627/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2627/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2627/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Canver CC, Voytovich MC. Resection of an unsuspected primary pulmonary choriocarcinoma. Ann Thorac Surg 1996;61:1249-51. [Crossref] [PubMed]
- Herai Y, Nishi K, Yamamoto H, et al. A case of primary choriocarcinoma of the mediastinum in a Japanese woman. Nihon Kokyuki Gakkai Zasshi 2006;44:384-8. [PubMed]
- Kageji T, Nagahiro S, Matsuzaki K, et al. Successful neoadjuvant synchronous chemo- and radiotherapy for disseminated primary intracranial choriocarcinoma: case report. J Neurooncol 2007;83:199-204. [Crossref] [PubMed]
- Shen FY, Zhao JK, Zhang HP, et al. Clues and traps of immunohistochemical diagnosis of pulmonary choriocarcinoma. Chinese Journal of Diagnostic Pathology 2020;27:854-8.
- Huang BX, Guo XB, Pan HX, et al. Expression of transcriptional factor GATA3 in trophoblastic tissues and its implications. Chinese Journal of Clinical and Experimental Pathology 2016;32:652-5.
- Gasparri R, Sedda G, Brambilla D, et al. When a Differential Diagnosis Is Fundamental: Choriocarcinoma Mimicking Lung Carcinoma. J Clin Med 2019;8:2018. [Crossref] [PubMed]
- Cho Y, Gorina S, Jeffrey PD, et al. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 1994;265:346-55. [Crossref] [PubMed]
- Baroni TE, Wang T, Qian H, et al. A global suppressor motif for p53 cancer mutants. Proc Natl Acad Sci U S A 2004;101:4930-5. [Crossref] [PubMed]
- Bullock AN, Fersht AR. Rescuing the function of mutant p53. Nat Rev Cancer 2001;1:68-76. [Crossref] [PubMed]
- Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010;2:a001008. [Crossref] [PubMed]
- Aubrey BJ, Strasser A, Kelly GL. Tumor-Suppressor Functions of the TP53 Pathway. Cold Spring Harb Perspect Med 2016;6:a026062. [Crossref] [PubMed]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003;3:11-22. [Crossref] [PubMed]
- Johnson DB, Lovly CM, Flavin M, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res 2015;3:288-95. [Crossref] [PubMed]
- Montazeri K, Bellmunt J. Erdafitinib for the treatment of metastatic bladder cancer. Expert Rev Clin Pharmacol 2020;13:1-6. [Crossref] [PubMed]
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3. [Crossref] [PubMed]



