
Case report: sequential use of almonertinib based on the EGFR exon 20 insertion mutation achieves long-term control for advanced non-small cell lung cancer patients
IntroductionOther Section
With the widespread application of next-generation sequencing (NGS), the incidence of rare epidermal growth factor receptor (EGFR) gene mutations, including exon 20 insertion (ex20ins) mutations, has continuously increased (1). EGFR ex20ins mutations are present in approximately 0.1% to 4% of all non-small cell lung cancer (NSCLC) and account for 4% to 12% of all EGFR mutations (2,3). The most common EGFR ex20ins mutation is A767_V769dup (2). No history of smoking, female sex and adenocarcinoma are common features of NSCLC with EGFR ex20ins mutations (3). The preferred first-line treatment for NSCLC patients with EGFR mutation-positive is EGFR-tyrosine kinase inhibitors (TKIs); however, ex20ins mutations are associated with primary resistance to EGFR-TKIs therapy (4). Therefore, patients with ex20ins mutations generally respond poorly to first- and second-generation EGFR-TKIs (5). Currently, the platinum and pemetrexed chemotherapy regimen remains the most effective first-line treatment for patients with EGFR ex20ins mutations, and there are no identified molecular targeted therapeutic drugs (5,6). Targeted therapy is currently widely applied, and the application of targeted drugs for the treatment of NSCLC patients with EGFR ex20ins mutations is of significant importance.
We report the case of a female patient with lung adenocarcinoma with an EGFR ex20ins mutation whose progression-free survival (PFS) was significantly prolonged with almonertinib treatment. This finding provides evidence in support of a new almonertinib-based regimen for the targeted treatment of patients with this class of rare mutations. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2728/rc).
Case presentationOther Section
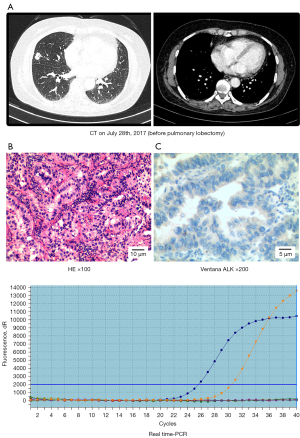
In July 2017, a 54-year-old Chinese woman who had never smoked and had no underlying disease was admitted to the hospital with cough and expectoration. Chest computed tomography (CT) showed a 12 mm × 7 mm mass in the middle lobe of the right lung and multiple minute nodules in the interpleural walking region of the right lobe (Figure 1A). Positron emission tomography-computed tomography (PET-CT) revealed a high level of 18F-fluorodeoxyglucose (FDG) avidity in the mass in the right middle lobe. The patient underwent a CT-guided percutaneous lung biopsy, and a pathological diagnosis of lung adenocarcinoma was determined (Figure 1B). In addition, brain magnetic resonance imaging (MRI) and whole-body bone scanning examinations were performed, and no distant metastasis was found. Video-assisted thoracoscopic wedge resection of the right middle lobe was performed under general anesthesia, and the tumor (15 mm × 10 mm) was found to be located in the middle lobe of the right lung and had invaded the lung visceral pleura, diaphragm and pleura without pleural effusion. Postoperative pathology suggested invasive adenocarcinoma, pT1bN0M1a stage IVa. Pathological analysis revealed that the tumor was negative for the anaplastic lymphoma kinase (ALK) fusion protein (Figure 1C). Medium-abundance mutations in EGFR ex20ins were detected in surgical specimens by quantitative real-time polymerase chain reaction (qRT-PCR, AccBio) (Figure 1D).
With the complete informed consent of the patient, systemic chemotherapy with the “pemetrexed (500 mg/m2, day 1) plus cisplatinum (25 mg/m2/day, days 1–3) plus bevacizumab (400 mg, day 1)” regimen was applied for 4 cycles, and “pemetrexed (500 mg/m2, day 1)” monotherapy was maintained for 1 cycle. One cycle is 21 days. During the treatment period, the patient’s condition was stable; however, the patient was not regularly treated and reviewed. Since August 2017, the patient has not undergone regular reexamination of CT after operation (Figure 2A). A CT scan up to August 2018 showed an increase in the level of pleural thickening and the number of small nodules (Figure 2B). The patient received 2 cycles of treatment with “pemetrexed (500 mg/m2, day 1, 21 days) plus bevacizumab (400 mg, day 1, 21 days)”. Thereafter, the patient did not undergo regular treatment or re-examination.
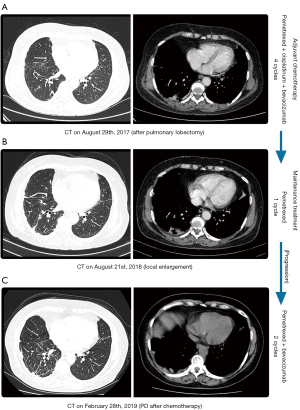
In February 2019, a CT scan (Figure 2C) indicated that the level of right pleural thickening (including interlobar fissure) had increased more than the anterior range. To determine whether the expression of new, sensitive target gene mutations had arisen after chemotherapy, the patient underwent a right pleural mass puncture biopsy, and the NGS still indicated lung adenocarcinoma with an EGFR ex20ins mutation (p.Ala767_Val769dup) (Figure 3A,3B). National Comprehensive Cancer Network (NCCN) guidelines suggest that treatment with the second-generation EGFR-TKI afatinib has better efficacy and yields a substantial PFS benefit. In March 2019, the patient regularly received afatinib (40 mg daily, p.o.) targeted therapy and was reviewed regularly. During this period, the patient did not complain of toxic symptoms. Tumor progression was observed after 3.2 months. It has been suggested that tumors with EGFR ex20ins mutations are more sensitive to poziotinib (a second-generation EGFR-TKI). In June 2019, the patient’s targeted therapy was changed to poziotinib (14 mg daily, p.o.) and her condition was stable during the regular treatment. During the treatment, the patient developed intermittent diarrhea and dry skin. However, in April 2020 (Figure 3C), a CT scan revealed obvious tumor progression. Compared with that observed on the previous CT scan, the right pleural membrane had thickened, multiple nodules had increased in size and right malignant pleural effusion had emerged, resulting in local atelectasis of the right lung.
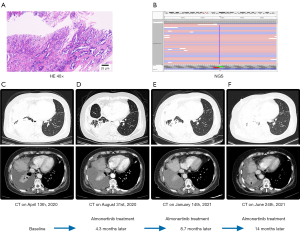
The patient refused chemotherapy, and considering the favorable treatment efficacy of the third-generation EGFR-TKI almonertinib in EGFR+ multiple targets inhibition, we initiated almonertinib (110 mg daily, p.o.) therapy in April 2020. After 2 months of treatment, the patient’s chest tightness had reduced and no adverse reactions arose. In August 2020 (Figure 3D), after 4.3 months of treatment, a CT scan revealed that the pleural effusion had reduced and that the tumor was stable. Subsequently, the patient was regularly reviewed. In January 2021, a CT scan indicated that the patient’s condition remained stable (Figure 3E). The patient then continued to take the medication regularly and was regularly reviewed. In June 2021, the patient developed chest tightness, shortness of breath and other symptoms, and a CT scan indicated tumor progression (Figure 3F). The overall PFS of almonertinib treatment was 14 months.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
DiscussionOther Section
With the identification of lung cancer-related genes and the development of corresponding targeted therapies, EGFR mutations have become an important predictor of the effectiveness of targeted therapy with EGFR-TKIs (7). NGS is an important detection method that can detect not only common drug-sensitive mutations, such as exon 19 deletion mutations and the exon 21-L858R point mutation, but also rare drug-resistant mutations such as ex20ins mutations (8,9). Rare and common mutations have similar clinicopathological characteristics, but common mutations respond well to EGFR-TKIs and rare mutations are associated with primary TKIs resistance (5,10,11). Thus, EGFR-TKIs has shorter PFS and lower disease control rates (DCRs) in patients with EGFR ex20ins mutations than those in patients with EGFR sensitive mutations (5,7,12). The preferred first-line regimen for NSCLC patients with sensitive EGFR mutations has changed chemotherapy to targeted therapy (13). Targeted therapy significantly improves the quality of life and prognosis of patients with NSCLC with drug-sensitive mutations compared with that of patients without targetable gene mutations who receive conventional chemotherapy (4). The incidence of EGFR ex20ins mutations is extremely low, and sufficient clinical trial evidence for their treatment is still lacking. However, TKIs treatment are critical, and studies have shown that irreversible TKIs can be effective for patients with such rare EGFR mutations (7). Second-generation EGFR-TKIs (afatinib and poziotinib) and third-generation EGFR-TKIs (almonertinib) irreversibly bind to mutant receptors (14). Almonertinib, as the third generation EGFR-TKI, can irreversibly bind to EGFR ATP binding region, and it is suitable for the treatment of NSCLC patients with disease progression and T790M drug resistance mutation positive after other EGFR-TKI treatment. These findings indicate that the application of these drugs is a potentially feasible therapeutic regimen for the targeted therapy of NSCLC with EGFR ex20ins mutations.
EGFR ex20ins is the most common rare mutation and may activate EGFR through rearranging the C-spiral; however, the affinity of this mutation for EGFR-TKIs is significantly inferior to that of other sensitive mutations (15). Thus, tumors with the ex20ins mutations are more invasive and have a worse prognosis than those with EGFR sensitive mutations and wild type EGFR (16). For patients with ex20ins mutations, the preferred treatment is often chemotherapy rather than EGFR-TKIs (3,5). Therefore, this patient was treated with “pemetrexed plus cisplatinum plus bevacizumab”, a first-line chemotherapy regimen for lung adenocarcinoma. It has been found that the PFS and OS of patients with ex20ins mutations undergoing chemotherapy were significantly shorter than those of patients with wild type EGFR (17). Due to limited clinical research, no determined molecular targeted drugs have been developed to address disease progression after chemotherapy. Previous studies have shown that the PFS after afatinib (a second-generation EGFR-TKI) treatment is significantly better than that after first-generation EGFR-TKIs treatment (11.3 vs. 3.6 months, P=0.03) (18). However, the PFS of this patient after afatinib treatment was only 3.2 months. Studies have shown that poziotinib has a good inhibitory effect not only on tumors that overexpress HER2 but also on those with EGFR ex20ins mutations (19). Poziotinib, which is small in size and has greater flexibility, can overcome the stereospecific blockade (size and configuration) of the drug binding site caused by the ex20ins mutation and thus can effectively combine with this mutated EGFR (10,20). Thus, tumors with ex20ins mutations are more sensitive to poziotinib than to other similar TKIs (afatinib and osimertinib), and can serve as an effective inhibitor of the EGFR ex20ins mutation (10). Unfortunately, this patient gradually developed drug resistance to poziotinib, with PFS for approximately 10.4 months. Almonertinib, a new third-generation EGFR-TKI, was modified on the basis of osimertinib, and cyclopropyl group was used instead of methyl group in indole ring. It irreversibly binds to the EGFR ATP binding region and produces irreversible inhibition of EGFR-mediated resistance and can be used to treat NSCLC patients with EGFR ex20ins mutations (14). We therefore treated the patient with almonertinib with good efficacy. The patient’s PFS with almonertinib treatment was 14 months. However, the specific mechanism of the drug’s effect needs to be further researched.
ConclusionsOther Section
This case report of a patient with a rare EGFR ex20ins (p.Ala767_Val769dup) mutation indicates that almonertinib treatment is a potential new option for patients with such mutations. Sequential therapy with EGFR-TKIs leads to longer patient survival times. To date, researchers have proposed many therapeutic regimens for the targeted therapy of tumors with EGFR ex20ins mutations, but a unified and exact approach has not yet been determined. Since different EGFR ex20ins mutations lead to different structures and functions, their complex mechanisms have not been clearly defined and distinct individual differences are present. Further studies should be conducted on the effectiveness of different irreversibly binding EGFR-TKIs for tumors with ex20ins mutations and the response of different subgroups of ex20ins mutations to the same EGFR-TKIs.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2728/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2728/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2728/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Watanabe M, Kawaguchi T, Isa S, et al. Ultra-Sensitive Detection of the Pretreatment EGFR T790M Mutation in Non-Small Cell Lung Cancer Patients with an EGFR-Activating Mutation Using Droplet Digital PCR. Clin Cancer Res 2015;21:3552-60. [Crossref] [PubMed]
- Fang W, Huang Y, Hong S, et al. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer 2019;19:595. [Crossref] [PubMed]
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [Crossref] [PubMed]
- Passaro A, Mok T, Peters S, et al. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J Thorac Oncol 2021;16:764-73. [Crossref] [PubMed]
- Xu J, Jin B, Chu T, et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in China. Lung Cancer 2016;96:87-92. [Crossref] [PubMed]
- Wu JY, Yu CJ, Shih JY. Effectiveness of Treatments for Advanced Non-Small-Cell Lung Cancer With Exon 20 Insertion Epidermal Growth Factor Receptor Mutations. Clin Lung Cancer 2019;20:e620-30. [Crossref] [PubMed]
- Zhang Q, Cui Y, Zhang J, et al. Comparison of the characteristics of uncommon epidermal growth factor receptor (EGFR) mutations and EGFR-tyrosine kinase inhibitor treatment in patients with non-small cell lung cancer from different ethnic groups. Exp Ther Med 2020;19:3513-20. [Crossref] [PubMed]
- Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet 2016;17:333-51. [Crossref] [PubMed]
- Chang Q, Qiang H, Qian J, et al. Epidermal Growth Factor Receptor Mutation Status and Response to Tyrosine Kinase Inhibitors in Advanced Chinese Female Lung Squamous Cell Carcinoma: A Retrospective Study. Front Oncol 2021;11:652560. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013;5:216ra177. [Crossref] [PubMed]
- Chen D, Song Z, Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther 2016;9:4181-6. [Crossref] [PubMed]
- Lei L, Wang WX, Zhu YC, et al. Potential mechanism of primary resistance to icotinib in patients with advanced non-small cell lung cancer harboring uncommon mutant epidermal growth factor receptor: A multi-center study. Cancer Sci 2020;111:679-86. [Crossref] [PubMed]
- Li M, Zhou CZ, Yang JJ, et al. The in cis compound EGFR mutations in Chinese advanced non-small cell lung cancer patients. Cancer Biol Ther 2019;20:1097-104. [PubMed]
- Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 2019;4:5. [Crossref] [PubMed]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288-300. [Crossref] [PubMed]
- Guo G, Li G, Liu Y, et al. Next-Generation Sequencing Reveals High Uncommon EGFR Mutations and Tumour Mutation Burden in a Subgroup of Lung Cancer Patients. Front Oncol 2021;11:621422. [Crossref] [PubMed]
- van Veggel B, de Langen AJ, Hashemi SMS, et al. Afatinib and Cetuximab in Four Patients With EGFR Exon 20 Insertion-Positive Advanced NSCLC. J Thorac Oncol 2018;13:1222-6. [Crossref] [PubMed]
- Koga T, Kobayashi Y, Tomizawa K, et al. Activity of a novel HER2 inhibitor, poziotinib, for HER2 exon 20 mutations in lung cancer and mechanism of acquired resistance: An in vitro study. Lung Cancer 2018;126:72-9. [Crossref] [PubMed]
- Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J Thorac Oncol 2018;13:1560-8. [Crossref] [PubMed]


