
Genomic profiling reveals non-small cell lung cancer with common mutations of EGFR exon 20 and exon 21: a case report
Introduction
Epidermal growth factor (EGF) was first discovered in neonatal rats in 1962 (1). Human epidermal growth factor receptor (EGFR) was isolated and purified in 1980 (2). With the progressions of research, EGFR and its downstream pathways have been continuously understood. Phosphatidylinosiyol-3-kinase (PI3K)/protein kinase B (PKB, also known as Akt) signaling pathway as a downstream pathway of EGFR is dysregulated in most human tumors. EGFR mainly stimulates the Ras protein after dimerization. Ras stimulation leads to the phosphorylation cascade and activates the PI3K/Akt signaling pathway which causes tumorigenesis and development. The discovery of somatic mutations in the Epidermal growth factor receptor (EGFR) in 2004 provided the basis for molecular typing of NSCLC. EGFR mutations mainly occur in the first four [18–21] exons of the intracellular TK region. There are currently more than 30 types of mutations in the tyrosine kinase (TK) region. Approximately 1% of patients with NSCLC have primary EGFR double mutations (3,4). According to research, for patients with non-small cell lung cancer (NSCLC) that cannot be surgically removed, the over survival (OS) of targeted therapy is higher than that of platinum-containing two-agent chemotherapy. And with the continuous updating of targeted drugs, new generation of targeted drugs can enter the brain through the blood-brain barrier, which has better curative effects on brain metastasis and longer drug resistance. It solves the problem that traditional chemotherapy drugs cannot enter the brain. Brain metastases can only be treated with radiotherapy or surgery. Now, the FDA has approved 6 drugs including erlotinib, gefitinib, icotinib, afatinib, dacomitinib, and osimertinib for the treatment of lung adenocarcinoma caused by EGFR gene mutations. In this study, we selected the patient who was stage IA of NSCLC. During the treatment, next-generation sequencing (NGS) gene detection revealed that the exon 20 of the EGFR gene p.R776S and the exon 21 of the EGFR gene p.L858R were co-mutated, and there were no other gene mutations. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2604/rc).
Case presentation
This study was approved by Ethics Committee of The Second Affiliated Hospital of Dalian Medical University. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. A 59-year-old Chinese female never-smoker/drinker, whose family members had no cancer history, with a lesion in the left upper lobe found by CT scan (Figure 1A,1B). The patient received single-incision video-assisted radical resection of pulmonary carcinoma in January 2019. Surgery and postoperative pathology showed (Figure 1C): moderately differentiated adenocarcinoma with 80% of acinar type and 20% of mural type (T1cN0M0, IA). The patient’s tumor tissues and peripheral blood samples were submitted for genetic testing by NGS, and we found a new type of double mutations of the EGFR gene. The results showed missense mutation in EGFR exon 21 c.2573T > G (p.L858R) with a mutant allelic frequency (MAF) of 6.3%, missense mutation EGFR exon 20 c.2326C > A (p.R776S) with a MAF of 4.7% and tumor mutational burden (TMB) of 2.3 mutations per Mb. No other genes were found to be mutated (Table 1 and Figure 2). Furthermore, RNA-seq technology confirmed the results of NGS (Figure 3).
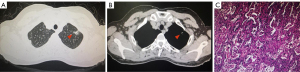
Table 1
| Genes | Alternations | Nucleotide change | Plasma | Tissue |
|---|---|---|---|---|
| EGFR | p.L858R | c.2573T>G | – | 6.3% |
| EGFR | p.R776S | c.2326C>A | – | 4.7% |
–: not detected. Each mutation is shown as the mutant allele frequency.
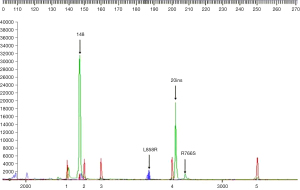
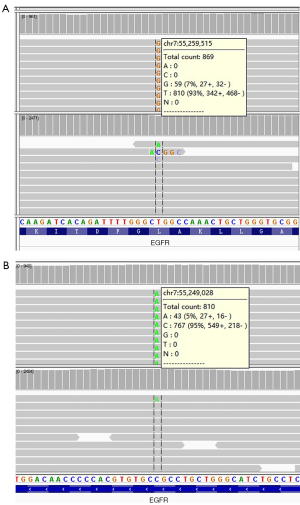
Discussion
Lung cancer is currently one of the most common cancers. The number of newly diagnosed patients and deaths of lung cancer in China account for 21.9% and 26.8% of the world total number (5). Lung cancer is the first most prevalent malignant tumor and the first leading cause of cancer-related deaths among the worldwide (6). The early clinical manifestations of lung cancer are relatively subtle and non-specific, while most patients are advanced when they were diagnosed. According to statistics, the 5-year survival rate of stage IA lung cancer is 73% (7).Therefore, early detection and early surgical treatment are recommended for lung cancer treatment.
This patient belongs to stage IA lung cancer. According to the NCCN guidelines, postoperative treatment is not required, there is only need of follow-up. Once there are signs of recurrence, targeted drug treatment can be used based on the comprehensive genetic test results after surgery. In patients with classic EGFR-mutated NSCLC, the response rate and median progression-free survival to targeted drugs were higher than those of chemotherapy (8). In recent years, the overall efficacy of chemotherapy and radiotherapy for NSCLC has reached a bottleneck (9). With the rapid development of precision medicine, targeted drugs targeting the EGFR gene have been successfully applied to the treatment of NSCLC, which has prolonged the survival time of patients and improved the quality of life. EGF was first found in the submandibular glands of mice. EGFR is a receptor for EGF cell proliferation and signaling, has TK activity, and is the expression product of the proto-oncogene C-erb-1 (HER-1). The composition is completed by 1,186 amino acid residues and belongs to the human chromosome 7p13-q22 region. It belongs to the multifunctional glycoprotein and is distributed on the outer surface of human keratinocytes, epithelial cells, glial cells, and fibroblasts. When EGFR is linked to the ligand, the TK component in the cell is activated, which causes the molecule to gradually phosphorylate and activate, forming a mitotic appearance. After phosphorylation of tyrosine, it can activate molecular sites, trigger signaling mechanisms, and activate downstream, etc. EGFR mutations has been well studied and is the second most common oncogenic driven mutation in NSCLC (10-13). These hot spot mutations of EGFR kinase domain (exon 18–21), increased the kinase activity of EGFR, can however lead to the hyperactivation of downstream pro-survival signal pathways and carcinogenesis of NSCLC cells. The EGFR is responsible for activation of main three intracellular signaling pathways involving PI3K/AKT. The mechanistic target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK), and interleukin 6 (IL-6)/janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) (13). Small molecule tyrosine kinase inhibitor (EGFR-TKI) which the mechanism of drug mainly inhibits the binding of ATP and TK binding domain by binding to the intracellular region of EGFR, preventing the receptor from phosphorylation. To achieve the role of blocking downstream signaling pathways. EGFR gene mutations are characteristic of tumor-specific and somatic hereditary changes, and their mutations are mainly in tumor lesions, but in normal tissue cells, this sign is basically absent.
At present, six drugs have been approved by the FDA for mutation of EGFR L858R, but the research mechanism of EGFR R776S is still unclear. More than one-half patients eventually develop disease progression treated with 1st-generation EGFR-TKIs (gefitinib and erlotinib) or 2nd-generation EGFR-TKIs (afatinib and dacomitinib) due to acquired resistance EGFR T790M (14). Osimertinib, a 3rd-generation EGFR TKI, selectively blocks the activated EGFR T790M mutation but acquired resistance is a growing clinical challenge. Other known mechanisms of resistance occur on non-T790M secondary EGFR mutations (1%), HER2 amplification (10–15%), MET amplification (5%), acquired mutations in the common downstream pathway (1%), or phenotypic transformation (10%) (15). In recent report, patients with atypical EGFR mutations had a shorter progression free survival (PFS) and overall survival (OS) than patients with classical EGFR mutations (L858R and/or Ex19del with or without T790M) (16). Interestingly, they found that exon 20 mutations are heterogenous in their response to EGFR TKIs and most exon 20 point mutations were P-loop and αC-helix compressing (PACC) mutations, which were more sensitive to the second-generation TKIs (17,18). Moreover, primary classic EGFR and acquired PACC mutations retained sensitivity to second-generation TKIs (16). This patient’s EGFR exon 20 and 21 exon double mutations can provide a new exploration for the new mechanism of EGFR-TKIs treatment, drug resistance, and combination therapy with chemotherapy or other targeted or even immune therapy.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2604/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2604/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by Ethics Committee of The Second Affiliated Hospital of Dalian Medical University. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem 1962;237:1555-62. [Crossref] [PubMed]
- Cohen S, Carpenter G, King L Jr. Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem 1980;255:4834-42. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Peng L, Song ZG, Jiao SC. Efficacy analysis of tyrosine kinase inhibitors on rare non-small cell lung cancer patients harboring complex EGFR mutations. Sci Rep 2014;4:6104. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin 2011;61:91-112. [Crossref] [PubMed]
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Fang MY, Wang SY, Zheng YB, et al. Prognostic and predictive significance of plasma hepatocyte growth factor and carcinoembryonic antigen in non-small lung cancer after surgery. Eur Rev Med Pharmacol Sci 2014;18:398-403. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [Crossref] [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [Crossref] [PubMed]
- Nagano T, Tachihara M, Nishimura Y. Mechanism of Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors and a Potential Treatment Strategy. Cells 2018;7:212. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-9. [Crossref] [PubMed]
- Robichaux JP, Le X, Vijayan RSK, et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 2021;597:732-7. [Crossref] [PubMed]
- Tanaka I, Morise M, Kodama Y, et al. Potential for afatinib as an optimal treatment for advanced non-small cell lung carcinoma in patients with uncommon EGFR mutations. Lung Cancer 2019;127:169-71. [Crossref] [PubMed]
- Yang JC, Schuler M, Popat S, et al. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J Thorac Oncol 2020;15:803-15. [Crossref] [PubMed]


