Inhibitory effect of lovastatin on human lung cancer cell proliferation by regulating the ERK1/2 and COX-2 pathways
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, of which deaths related to metastasis account for 90% of all lung cancer deaths. Lung cancer treatment ranges from traditional surgery, chemotherapy, and radiotherapy to targeted therapy, immunotherapy, and combination therapies (1-3). Researchers have made new breakthroughs in the field of tumor treatment drugs, being of great significance to cancer treatment. Traditionally, lovastatin, an inhibitor of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, has been used to lower cholesterol; however, growing evidence has documented that lovastatin can prevent tumor cell proliferation and metastasis, and exerts effects on cancer cell apoptosis. Consequently, lovastatin has the potential to be an anti-tumor adjuvant drug (4,5).
Results from studies have yielded conclusive evidence that lovastatin has inhibitory effects on tumor proliferation. Laezza et al. demonstrated that lovastatin suppressed the extracellular signal-regulated kinase (ERK) 1/2 signaling pathway, thereby inducing the apoptosis of K-RAS-transformed thyroid cells. The anti-tumor efficacy of lovastatin has been confirmed to be related to oxidative stress, in which the p38 mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-kB) pathways were involved in the apoptotic effect of lovastatin (6). ERK1/2, as a member of the MAPK family, is a protein kinase with serine/threonine residues, mainly transmitting extracellular signals to the nucleus through phosphorylation activation and subsequently activating nuclear transcription factors. The activated ERK1/2, namely p-ERK1/2, regulates the transcription of related genes by phosphorylating nuclear transcription factors (such as c-FOS, c-JUN, and NF-ĸB), protein kinases, and substrates. The abnormal expression of ERK1/2 can lead to the apoptosis of normal and tumor cells, inhibiting cell proliferation (7,8). Other signaling pathway molecules abnormal expression exist, besides ERK1/2. Cyclooxygenase-2 (COX-2), being a rate-limiting enzyme in the synthesis of prostaglandins (PGs), is expressed in tumor cells on the basis of oncogene stimulation and plays as a pivotal role in pathophysiological processes. COX-2 overexpression can facilitate tumor growth by means of inhibiting cell apoptosis and promoting cell proliferation (9-11).
Previous experimental results showed that lovastatin has a significant inhibitory effect on the proliferation of human lung adenocarcinoma cells, but the mechanism remains unclear. In this study, we performed a preliminary exploration of the inhibitory effect of lovastatin on the proliferation of human lung adenocarcinoma cells and ascertained its molecular mechanism. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-346/rc).
Methods
Cell culture and treatment
The human lung adenocarcinoma cells, A549 cells were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). A549 cells were treated with different lovastatin concentrations of 5, 10, 15, 25, and 50 µM for 24 h when the confluency reached about 70%. In intervention experiments, cells were inoculated and treated with 1 µg/mL LPS for 8 h + 5 mM ATP for the last half an hour or phosphate buffered saline (PBS) for 8 h when the confluency reached about 60%. After the 8 h-treatment, the cells were treated with 50 µM lovastatin or solvent for 24 h. The cells were harvested for further experiments when the treatment was completed.
Cell viability assay
A549 cell viability was measured using the Cell Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan) following cell culture and drug treatment. The assay was performed according to the manufacturer’s recommended protocol. The absorbance of formazan was evaluated at 450 nm using a Bio-Tek Elx 800 microplate reader (Bio-Tek, VT, USA).
Western blotting
Protein was extracted from A549 cells. Cellular proteins were extracted using RIPA lysis buffer (Beyotime, Shanghai, China) containing 1% phosphatase and protease inhibitors, then quantified with the BCA protein assay kit (Beyotime, Shanghai, China). Total protein was separated by SDS-PAGE and transferred onto a PVDF membrane (Millipore, Massachusetts, USA). After blocking with 5% low-fat milk at room temperature for 1 h, the membranes were incubated with primary antibodies (1:1,000) overnight at 4 ℃. The following day, membranes were incubated with secondary antibodies (1:2,000) for 1 h at room temperature. The resulting immunoreactive bands were visualized using a chemiluminescence system (Tanon-5200; Tanon Science & Technology Co., Ltd., Shanghai, China) and analyzed with ImageJ software 1.51 (NIH, MD, USA). Relative protein levels were determined with β-Actin as the internal reference.
The following is the detailed information of primary antibodies. ERK1/2 (ab184699 Abcam, Massachusetts, USA), pERK1/2 (ab223500, Abcam), c-JUN (ab31419, Abcam), p-c-JUN (ab32385, Abcam), Cox-2 (ab62331, Abcam), BCL-2(ab196495, Abcam), Bax (ab53154, Abcam) and β-Catenin (ab32575, Abcam).
Immunofluorescence staining
A549 cells slices were fixed using 4% paraformaldehyde, then blocked and permeabilized with 1% bovine serum albumin and PBS. Cells were incubated in primary antibody diluent overnight at 4 ℃. Cell nuclei were labeled with 4’,6-diamidino-2-phenylindole for 5 min and then washed 3 times. The slides were covered with Antifade Mounting Medium (Beyotime, Shanghai, China) and coverslips, and were subsequently imaged and analyzed using Image-Pro Plus 6 software (Media Cybernetics, Rockville, MD, USA).
Colony formation assay
After different treatment as previously described, A549 cells were subjected to clonogenic assays. Approximately 500 cells were seeded into individual wells of a 6-well plate in triplicate and maintained with the indicated treatment (LPS+ATP or different concentrations of lovastatin). After 7 days, cells were fixed and stained with methylene blue, and viable colonies (50 cells) were counted under a microscope. Data were fitted to the linear quadratic equation using GraphPad Prism version 5 (GraphPad, La Jolla, CA, USA) (12).
Statistical analysis
Experimental data were analyzed with SPSS 25.0 (SPSS, Chicago, IL, USA) and data were expressed as the mean ± SEM of at least 3 independent replicates. One-way ANOVA or two-way independent samples t-test were used. A value of P<0.05 was considered significant.
Results
Lovastatin suppressed A549 cell proliferation and modulated protein expression in a dose-dependent manner
To investigate the signaling pathways affected by lovastatin, we firstly determined the viability of A549 cells treated with different concentrations of lovastatin (0, 5, 10, 15, 25, 50 µM) for 24 h (Figure 1A). The viability of A549 cells cultured with lovastatin for 24 h was markedly reduced, and this effect was dose-dependent (P<0.05). We subsequently determined the expression of crucial proteins in A549 cells after treatment with lovastatin which were involved in the regulation of cell proliferation and apoptosis.
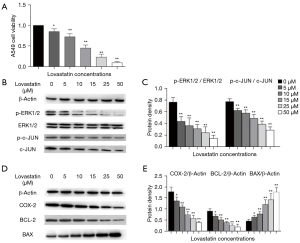
The results showed that p-ERK1/2/ERK1/2 and p-c-JUN/c-JUN expression levels were obviously lower than those of the control group, suggesting that lovastatin can inhibit the proliferation of A549 cells via suppressing the activation of ERK1/2 and c-JUN (Figure 1A,1B). The protein levels of COX-2 and BCL-2 were reduced, while BAX was significantly elevated, thus indicating that lovastatin can induce the apoptosis of A549 cells (Figure 1C-1E). Of note, the effect of 50 µM lovastatin on the expression of the above proteins was the most obvious. Consequently, we adopted the concentration of 50 µM lovastatin to conduct the subsequent intervention experiments, further proving that lovastatin can suppress the growth of A549 cells by modulating the ERK1/2 and COX-2 pathways.
Lovastatin inhibited A549 cell proliferation and regulated protein expression even after LPS+ATP stimulation
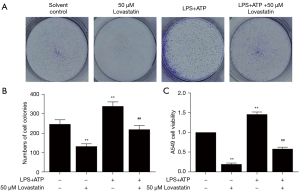
To confirm that lovastatin can suppress A549 cell proliferation through the ERK1/2 and COX-2 pathways, we stimulated A549 cell proliferation using 1 µg/mL LPS for 8 h and 5 mM ATP for the last half an hour. In contrast to the control group and LPS+ATP group, a decreased number of cell colonies and cell viability were found after LPS+ATP+50 µM lovastatin treatment (Figure 2). The colony formation and cell viability assay demonstrated that lovastatin, despite LPS+ATP stimulation, can still suppress A549 cell growth.
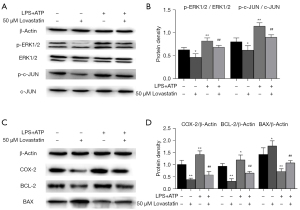
As for protein expression, p-ERK1/2/ERK1/2 and p-c-JUN/c-JUN expression levels in A549 cells treated with 50 µM lovastatin were significantly lower compared with the control group (P<0.05), but were elevated in the LPS+ATP intervention group (P<0.01). In contrast to the LPS+ATP group, surprisingly, p-ERK1/2/ERK1/2 and p-c-JUN/c-JUN expression levels after LPS+ATP+50 µM lovastatin intervention were significantly reduced (P<0.01) (Figure 3A,3B). Likewise, COX-2 and BCL-2 expression revealed a decreased trend similar to that of p-ERK1/2/ERK1/2 and p-c-JUN/c-JUN protein levels (P<0.01), whereas BAX expression displayed an increased trend (P<0.05) (Figure 3C,3D). COX-2 and BCL-2 protein levels in the LPS+ATP group were significantly higher than those in the control group (P<0.01), whereas their expression levels after LPS+ATP+50 µM lovastatin treatment were significantly lower than in the LPS+ATP group (P<0.01). The expression of BAX exhibited an opposite trend, as BAX expression after LPS+ATP+50 µM lovastatin intervention was elevated compared with the LPS+ATP group.
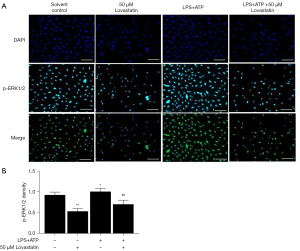
Inhibition of lovastatin on the p-ERK1/2 and COX-2 pathways in A549 cells
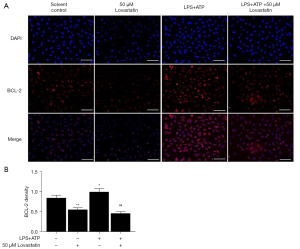
In order to explore whether lovastatin inhibited A549 cell proliferation via regulation of the ERK1/2 and COX-2 pathways, we detected the protein expression of p-ERK1/2 and BCL-2 by immunofluorescence in the subsequent intervention experiment. Lower fluorescence-intensity of p-ERK1/2 and BCL-2 in the 50 µM lovastatin group was observed compared with the control group (P<0.01) (Figures 3,4). Conversely, higher-intensity was observed in the LPS+ATP group (P<0.05). Meanwhile, the intensity of A549 cells after treatment with LPS+ATP+50 µM lovastatin was significantly down-regulated compared to the LPS+ATP group (P<0.01) (Figures 4,5). These findings indicate that lovastatin can suppress the activation of the p-ERK1/2 and BCL-2 signaling pathways in A549 cells, thereby controlling cancer cell growth.
Discussion
Lovastatin is an inhibitor of HMG-CoA reductase in the process of cholesterol synthesis. It can effectively suppress cholesterol synthesis and is therefore applied to hypercholesterolemia treatment in the clinic (13,14). Besides its lipid-lowering function, there is evidence to suggest that lovastatin can inhibit tumor cell proliferation, angiogenesis, invasion, and metastasis, and can induce apoptosis (15,16). Indeed, a series of studies have verified that lovastatin combined with a myriad of classical anti-cancer drugs displayed a synergistic inhibitory role, enhancing the anti-tumor effect (17-19). Therefore, it is of great importance to explore the mechanism of lovastatin’s therapeutic effect on tumors.
Previous studies found that statin use may diminished lung cancer mortality. A retrospective case-control study has reported an association of statin use for >6 months with a 55% risk reduction (4). In vitro activity Hawk et al. demonstrated that lovastatin inhibited lung tumor formation induced by 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) at an early promotional stage and promoted the apoptosis of NCI-H125, NCI-H292, NCI-H441, NCI-H460, and NCI-H661 lung epithelial cells (20). After treating non-small cell lung cancer (NSCLC) with different concentrations of lovastatin (0, 2.5, 5, 10, and 20 µM), Zhang et al. reported that lovastatin markedly and consistently suppressed tumor cell proliferation by down-regulating minichromosome maintenance 2 (MCM2) expression caused by an increase in p-JNK (21). Consistent with previous studies, we revealed that lovastatin had a suppressive effect on A549 cell growth, displaying a concentration-dependent response. These findings indicate that lovastatin can not only suppress tumor cell proliferation, but also stimulate apoptosis (22,23).
However, much of the prior research up to now has not fully clarified the mechanism of the suppressive effect of lovastatin on tumors. Dimitroulakos et al. showed combining lovastatin and gefitinib enhanced inhibition and cooperative cytotoxicity in a variety of cell lines that included all eight squamous cell carcinomas by inhibiting EGF-induced EGFR autophosphorylation (24). Di Bello et al. achieved rapidly proliferating of cancer cells by inhibited lipid metabolism and the mevalonate pathway with lovastatin (25). The current study was designed to determine the underlying mechanisms by means of measuring ERK1/2 and COX-2 signaling pathway-associated proteins. According to these data, lovastatin led to a notable reduction of p-ERK1/2 and p-c-JUN expression and a considerable elevation of BAX expression in A549 cells with increasing lovastatin concentrations. This mechanism of modulating the ERK1/2 and COX-2 signaling pathways may be responsible for lovastatin’s regulatory effects on the proliferation of A549 cells. Therefore, the pathway through which lovastatin suppresses A549 cell proliferation requires further investigation.
It is reasonable to believe that the activation of ERK1/2 plays an active effect on c-JUN, stimulating cell proliferation and protecting cells from apoptosis (7). As a result, the expression levels of p-ERK1/2/ERK1/2 and p-c-JUN/c-JUN are an indication of cell proliferative capacity. Initially, the findings of cell viability and the colony formation assay confirmed that lovastatin can modulate A549 cell proliferation in spite of LPS+ATP stimulation, and a dose-response relationship of the suppressive effects was exhibited. Moreover, what strikingly stood out in the western blotting and immunofluorescence assays was the steady decline of p-ERK1/2/ERK1/2 and p-c-JUN/c-JUN protein levels in A549 cells after treatment with lovastatin. In accordance with the present results, a previous study by Yu et al. demonstrated that lovastatin attenuated cancer cell proliferation via the ERK1/2 pathway (26).
There has been a wide consensus that cell apoptosis plays a paramount role in tumor formation and development. Several laboratory studies of both premalignant and malignant tissues have provided evidence that increased expression of COX-2 can facilitate cell proliferation and protect cancer cells from apoptosis, thereby expediting tumor growth (27-29). Inhibition of COX-2 in animal models and epidemiological studies have confirmed its significant implications for cancer treatment or prevention in general. Sheng et al. reported that a COX-2 inhibitor, SC-85125, suppressed the growth of HCA-7 colon cancer cells, in which COX-2 was highly expressed (30). Our experimental results were similar to other studies in that lovastatin inhibited the level of COX-2 expression in A549 cells, thus inducing cancer cell death.
Although the mechanism of COX-2 pathway suppression in inhibiting cell apoptosis is not yet clear, one explanation may be related to the induction of BCL-2 expression by PGs (30). BCL-2 is an apoptosis suppressor which can prolong cell survival time and prevent cell apoptosis, which play a major role in the- negative feedback regulation of the immune response to apoptosis in the immune system regulation (31,32). BAX can form heterodimers with BCL-2, and consequently, it inhibits BCL-2 and jointly determines the process of cell apoptosis. Therefore, BAX is considered as one of the most important pro-apoptotic proteins (33). The results obtained from our analysis confirmed that the protein level of BCL-2 in A549 cells showed a decreasing trend with increasing concentrations of lovastatin compared to the control group, while the level of BAX increased. Lovastatin induced underexpression of COX-2 and BCL-2 as well as overexpression of BAX in A549 cells, thus activating cell apoptosis.
In the subsequent experimental analysis, A549 cells were exposed to proinflammatory stimuli (LPS+ATP) to accelerate cell proliferation, and the inhibiting action and mechanism of lovastatin was further explored. After intervention with LPS+ATP, however, lovastatin still manifested the same suppressive effect on A549 cells as the above experimental results. Interestingly, the protein levels of ERK1/2 pathway proteins p-ERK1/2 and p-c-JUN and COX-2 pathway proteins BCL-2 and BAX were modulated by lovastatin to keep tumor cell growth under control and facilitate apoptosis on the basis of LPS+ATP-induced proliferation. From this part of the study, it was evident that lovastatin had a suppressive effect on lung cancer cell proliferation by modulating the ERK1/2 and COX-2 pathways, despite LPS+ATP advancing cancer cell development. There are already some target drugs for lung cancer with lovastatin. Lovastatin led to mutant p53 protein degradation by activating a caspase-dependent apoptotic pathway and decreased motility in lung cancer cells possessing p53 missense mutations. The same drug hampered viability, stemness, tumor growth, and metastasis in pancreatic cells, via the inhibition of the Shh signaling leading to enhanced efficacy of gemcitabine treatment. Thus, we believe that ERK1/2 and COX-2 pathways has the potential to be used in lung cancer treatment.
This project was undertaken to investigate the effect of lovastatin on lung cancer cells and determine its anti-tumor mechanism. The most obvious finding to emerge from this study is that lovastatin could suppress lung cancer cell proliferation by regulating the ERK1/2 and COX-2 pathways. The evidence from this study suggests that lovastatin is a promising anti-tumor agent, and our findings also provided a deeper insight into its anti-tumor mechanism, warranting further preclinical and human studies. The principal limitation of this analysis was that only one lung cancer cell line was employed, and selective inhibitors of the ERK1/2 and COX-2 pathways were not included. Further investigation and experimentation regarding the impact of lovastatin on lung cancer would be worthwhile.
Acknowledgments
Funding: This work was supported by the Scientific Research Project of Health and Family Planning Industry in Hainan Province, China (No. 19A200036); Hainan Province Clinical Medical Center.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-346/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-346/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-346/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu HY, Yang FL, Li LH, et al. Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Sci Rep 2018;8:17956. [Crossref] [PubMed]
- Valdes M, Nicholas G, Goss GD, et al. Chemotherapy in recurrent advanced non-small-cell lung cancer after adjuvant chemotherapy. Curr Oncol 2016;23:386-90. [Crossref] [PubMed]
- Al-Tarakji M, Feilchenfeldt J, Haidar A, et al. Rare occurrence of metastasis from lung cancer to the anus: case report and review of the literature. World J Surg Oncol 2016;14:157. [Crossref] [PubMed]
- Khurana V, Bejjanki HR, Caldito G, et al. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest 2007;131:1282-8. [Crossref] [PubMed]
- Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst 2006;98:1819-25. [Crossref] [PubMed]
- Laezza C, Fiorentino L, Pisanti S, et al. Lovastatin induces apoptosis of k-ras-transformed thyroid cells via inhibition of ras farnesylation and by modulating redox state. J Mol Med (Berl) 2008;86:1341-51. [Crossref] [PubMed]
- Warmka JK, Solberg EL, Zeliadt NA, et al. Inhibition of mitogen activated protein kinases increases the sensitivity of A549 lung cancer cells to the cytotoxicity induced by a kava chalcone analog. Biochem Biophys Res Commun 2012;424:488-92. [Crossref] [PubMed]
- Li G, He Y, Yao J, et al. Angelicin inhibits human lung carcinoma A549 cell growth and migration through regulating JNK and ERK pathways. Oncol Rep 2016;36:3504-12. [Crossref] [PubMed]
- Herrero A, Benedicto A, Romayor I, et al. Inhibition of COX-2 Impairs Colon Cancer Liver Metastasis through Reduced Stromal Cell Reaction. Biomol Ther (Seoul) 2021;29:342-51. [Crossref] [PubMed]
- Ramer R, Walther U, Borchert P, et al. Induction but not inhibition of COX-2 confers human lung cancer cell apoptosis by celecoxib. J Lipid Res 2013;54:3116-29. [Crossref] [PubMed]
- Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci 2000;37:431-502. [Crossref] [PubMed]
- Good NM, Moore RS, Suriano CJ, et al. Contrasting in vitro and in vivo methanol oxidation activities of lanthanide-dependent alcohol dehydrogenases XoxF1 and ExaF from Methylobacterium extorquens AM1. Sci Rep 2019;9:4248. [Crossref] [PubMed]
- McCarty MF, O'Keefe JH, DiNicolantonio JJ. Red Yeast Rice Plus Berberine: Practical Strategy for Promoting Vascular and Metabolic Health. Altern Ther Health Med 2015;21:40-5. [PubMed]
- Palomino-Morales R, Perales S, Torres C, et al. Effect of HMG-CoA Reductase Inhibition on Vascular Smooth Muscle Cells Extracellular Matrix Production: Role of RhoA. Curr Vasc Pharmacol 2016;14:345-52. [Crossref] [PubMed]
- Mahmoud AM, Aboul-Soud MA, Han J, et al. Transcriptional profiling of breast cancer cells in response to mevinolin: Evidence of cell cycle arrest, DNA degradation and apoptosis. Int J Oncol 2016;48:1886-94. [Crossref] [PubMed]
- Zheng C, Yan S, Lu L, et al. Lovastatin Inhibits EMT and Metastasis of Triple-Negative Breast Cancer Stem Cells Through Dysregulation of Cytoskeleton-Associated Proteins. Front Oncol 2021;11:656687. [Crossref] [PubMed]
- Wu D, Chen Y, Wen S, et al. Synergistically Enhanced Inhibitory Effects of Pullulan Nanoparticle-Mediated Co-Delivery of Lovastatin and Doxorubicin to Triple-Negative Breast Cancer Cells. Nanoscale Res Lett 2019;14:314. [Crossref] [PubMed]
- Li Y, Chen S, Zhu J, et al. Lovastatin enhances chemosensitivity of paclitaxel-resistant prostate cancer cells through inhibition of CYP2C8. Biochem Biophys Res Commun 2022;589:85-91. [Crossref] [PubMed]
- Siddiqui RA, Harvey KA, Xu Z, et al. Characterization of lovastatin-docosahexaenoate anticancer properties against breast cancer cells. Bioorg Med Chem 2014;22:1899-908. [Crossref] [PubMed]
- Hawk MA, Cesen KT, Siglin JC, et al. Inhibition of lung tumor cell growth in vitro and mouse lung tumor formation by lovastatin. Cancer Lett 1996;109:217-22. [Crossref] [PubMed]
- Zhang X, Teng Y, Yang F, et al. MCM2 is a therapeutic target of lovastatin in human non-small cell lung carcinomas. Oncol Rep 2015;33:2599-605. [Crossref] [PubMed]
- Sanli T, Liu C, Rashid A, et al. Lovastatin sensitizes lung cancer cells to ionizing radiation: modulation of molecular pathways of radioresistance and tumor suppression. J Thorac Oncol 2011;6:439-50. [Crossref] [PubMed]
- Walther U, Emmrich K, Ramer R, et al. Lovastatin lactone elicits human lung cancer cell apoptosis via a COX-2/PPARγ-dependent pathway. Oncotarget 2016;7:10345-62. [Crossref] [PubMed]
- Dimitroulakos J, Lorimer IA, Goss G. Strategies to enhance epidermal growth factor inhibition: targeting the mevalonate pathway. Clin Cancer Res 2006;12:4426s-31s. [Crossref] [PubMed]
- Di Bello E, Zwergel C, Mai A, et al. The Innovative Potential of Statins in Cancer: New Targets for New Therapies. Front Chem 2020;8:516. [Crossref] [PubMed]
- Yu F, Gajendran B, Wang N, et al. ERK activation via A1542/3 limonoids attenuates erythroleukemia through transcriptional stimulation of cholesterol biosynthesis genes. BMC Cancer 2021;21:680. [Crossref] [PubMed]
- Buranabunwong N, Ruangrungsi N, Chansriniyom C, et al. Ethyl acetate extract from Glycosmis parva leaf induces apoptosis and cell-cycle arrest by decreasing expression of COX-2 and altering BCL-2 family gene expression in human colorectal cancer HT-29 cells. Pharm Biol. 2015;53:540-547. [Crossref] [PubMed]
- Garg R, Blando JM, Perez CJ, et al. COX-2 mediates pro-tumorigenic effects of PKCε in prostate cancer. Oncogene 2018;37:4735-49. [Crossref] [PubMed]
- Xiang L, Wang W, Zhou Z, et al. COX-2 promotes metastasis and predicts prognosis in gastric cancer via regulating mTOR. Biomark Med 2020;14:421-32. [Crossref] [PubMed]
- Sheng H, Shao J, Morrow JD, et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 1998;58:362-6. [PubMed]
- Lang-Rollin I, Maniati M, Jabado O, et al. Apoptosis and the conformational change of Bax induced by proteasomal inhibition of PC12 cells are inhibited by bcl-xL and bcl-2. Apoptosis 2005;10:809-20. [Crossref] [PubMed]
- Danial NN, Gimenez-Cassina A, Tondera D. Homeostatic functions of BCL-2 proteins beyond apoptosis. Adv Exp Med Biol 2010;687:1-32. [Crossref] [PubMed]
- Brajusković G, Orolicki SV, Cerović S, et al. Bcl-2 and Bax protein interaction in B-lymphocytes of peripheral blood in patients with chronic lymphocytic leukemia. Vojnosanit Pregl 2005;62:357-63. [Crossref] [PubMed]



