
Does neoadjuvant chemoradiotherapy increase the effect of lateral lymph node dissection on urogenital function?
Introduction
Colorectal cancer (CRC), with morbidity and mortality ranking fourth and second, respectively, is one of the most common cancers of the digestive system in the world (1). To date, the gold standard treatment for mid-low rectal cancer is total mesorectal excision (TME) (2). However, whether to perform lateral lymph node dissection (LLND) on the basis of TME is still a controversial issue worldwide. This might be due to the difference between Japan and Western countries in terms of the definition of lateral pelvic lymph node metastasis (LPNM). Japanese guidelines consider LPNM to be a local disease; thus, LLND is widely carried out (3,4). Nevertheless, LLND has not yet gained wide acceptance in Western countries. Western guidelines define LPNM as a marker of distant disease status, which is recognized as an advanced disease and leads to the use of neoadjuvant chemoradiotherapy (nCRT) for any enlarged nodes or locally-advanced primary tumors, and LLND would be added if lateral pelvic lymph nodes persist after nCRT (5-7). Several recent studies revealed that nCRT could decrease the size and purify suspected lateral pelvic lymph nodes clinically, which could alter the indications for LLND (8-10). Based on previous studies, in China, nCRT might be recommended to patients with suspected lateral pelvic lymph nodes initially, and then the decision of whether to perform LLND should take into account the lateral pelvic lymph node response to nCRT (8,11,12).
nCRT and LLND have effectively reduced local recurrence and significantly improved the survival rate (13,14). However, the LLND technique totally mobilizes the pelvic nerve plexuses, making LLND is closely correlated with urogenital complications (15-17). In addition, a number of studies demonstrated that nCRT might impair urogenital function, possibly because nCRT damages nerve function directly and causes fibrosis in sphincters (18,19). Nevertheless, there is no consensus regarding the extra risk of nCRT on urogenital function in rectal cancer undergoing LLND. In this study, we aimed to evaluate the effect of nCRT on the urogenital disorder of patients who underwent TME + LLND as well as determine the risk factors for urogenital dysfunction. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-87/rc).
Methods
Patients
Between January 2015 and December 2020, a total of 145 patients who underwent TME plus LLND at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College for mid-low rectal cancer (located below the peritoneal reflection) were enrolled. The inclusion criteria were as follows: (I) pathologically diagnosed rectal adenocarcinoma; (II) a tumour less than 12 cm from the anus on the lower edge; (III) patients diagnosed with stage II/III tumor-node-metastasis by MRI before treatment; and (IV) patients with suspected LPNM based on MRI. The exclusion criteria were as follows: (I) evidence of distant metastasis; (II) patients diagnosed with other malignant tumours; (III) patients with incomplete medical records; (IV) patients who have lost follow-up after surgery; (V) patients with partial vaginectomy or partial prostatectomy; (VI) patients who suffered from urogenital function before treatment. Finally, a total of 106 people were included in the study and they were divided into the nCRT+ group (n=51) and the nCRT− group (n=55).
Two imaging experts specializing in CRC diagnosed the illness as clinical LPNM based on MRI before treatment. The indications of LLND were described in our previous study (12): ≥8 mm in the short diameter, inhomogeneous or intense enhancement and irregular shape or rough edges. Meeting one or more of the above criteria can be diagnosed as LPNM and treated with LLND. The American Joint Committee on Cancer (AJCC, the 8th edition) staging system was used for tumor staging. The basic indication for nCRT at our hospital was locally advanced (cT3–4 or cN+) rectal cancer, but in some patients nCRT was abandoned at the discretion of the surgeon and oncologist considering the patient’s wishes and economic situation. Indications for nCRT were determined jointly by the physician and the patient, and baseline data were balanced for both groups to minimize selection bias. The nCRT+ group received radiotherapy (50 Gy/25 f/2 Gy) in combination with capecitabine at a dose of 825 mg/m2 twice daily on all treatment days, while the nCRT− group received surgery alone. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (NCC2017-YZ-026, 17 October 2017) and individual consent for this retrospective analysis was waived.
Surgical procedures
All patients were placed in the modified lithotomy position, and the majority of patients underwent laparoscopic surgery with a 5-port technique. During the operation, the abdominal cavity was explored routinely to confirm that there was no danger of organ invasion or implantation metastasis. TME was performed along the space between the pelvic fascia wall layer and the visceral layer based on strict principles (2). According to the classification of pelvic lateral lymph nodes by the Japan Colorectal Cancer Research Association (JSCCR, 2nd English edition), they can be divided into the following five regions: the common iliac node, distal internal iliac nodes, proximal internal iliac nodes, external iliac node, and obturator node. After TME, the unilateral or bilateral common iliac node, external iliac node, internal iliac and obturator lymph node were dissected in an orderly manner, and care was taken to identify and preserve the pelvic autonomic nerve. All operation were performed by colorectal surgeons with more than 20 years of experience in laparoscopic surgery.
Follow-up
Outpatient re-examination was performed every 3 months, including physical examination, tumour biomarkers (CEA and CA-199), colonoscopy, and CT scans of the thorax, abdomen and pelvis.
Functional outcome assessment
As part of the follow-up strategy, all surviving patients received anonymous and confidential questionnaires in February 2021, at least 6 months after surgery. The International Prostatic Symptom Score (IPSS) was used to assess urinary symptoms, which included frequency, nocturia, intermittency, urgency, straining, weak stream, and incomplete bladder emptying. The grading system uses a 0 to 5 scale, with 0 reflecting normal function and 5 reflecting bad function (0, not at all/not applicable; 1, less than 1 time in 5; 2, less than half the time; 3, approximately half the time; 4, more than half the time; 5, practically always). IPSS scores were used to classify urinary function (normal, 0–7 points; moderate dysfunction, 8–19 points; severe dysfunction, 20–35 points), the last two are referred to as urinary dysfunction.
The International Questionnaire of Erectile Function (International Index Erectile Function, IIEF) was used to examine male sexual function, including erectile function and ejaculation function. Similarly, all questions employ 5-point verbal and numerical scales to rate the existence or absence of a symptom, as well as its severity (0, never or only sometimes; 1, never or only occasionally; 2, less than half the time; 3, sometimes or half the time; 4, more than half the time; 5, practically always). IIEF scores were used to categorize sexual function (normal, >21 points; mild dysfunction, 16–20 points; moderate dysfunction, 11–15 points; severe dysfunction, 5–10 points), and the last three were referred to as sexual dysfunction. To assess subjective functional decrease, the questions included pre- and postoperative urogenital function. Erectile function was not assessed in patients with sexual inactivity (scores ranging from 0 to 4). Female patients were excluded from the analysis of sexual dysfunction because of poor clinical records.
Statistical analyses
The data were analysed by the SPSS26.0 statistical software package for Windows (IBM Crop., Armonk, NY, USA). The quantitative variables were expressed as the mean ± standard deviation (SD) and were compared by t-test. Categorical data were expressed as proportions and were compared by χ2 or Fisher’s exact test. Multiple logistic regression (forward LR) was used to determine the associations of clinical factors with outcomes. P<0.05 indicated a statistically significant difference.
Results
Study population
Between January 2015 and December 2020, a total of 145 patients with rectal cancer underwent surgery in the National Cancer Center. However, 39 patients were excluded from our study, and the remaining 106 patients were stratified into the nCRT+ group (n=51) and the nCRT− group (n=55) (Figure 1). The median follow-up time was 28 months.

The characteristics of all patients and findings during surgery procedures were listed in Table 1. No statistically significant difference was determined between the two groups with regard to sex, age, body mass index (BMI), tumour distance from the anal verge, ASA score or TNM status. The mean tumour size was 2.8 cm in the nCRT+ group and 4.1 cm in the nCRT− group (P=0.000). A statistically significant difference was found regarding the type of operation (P=0.022). Of the 51 patients in the nCRT+ group, 17 (33.3%) underwent low anterior resection, 33 (64.7%) underwent abdominoperineal resection, and only one patient (2.0%) underwent Hartmann’s procedure; of the 55 patients in the other group, 31 (56.4%) underwent low anterior resection, 22 (40.0%) underwent abdominoperineal resection, and 2 (3.6%) underwent Hartmann’s procedure. Additionally, the operation period from the beginning of anaesthetic induction to patient awakening in the nCRT+ group was significantly longer than that in the nCRT− group (296.6 vs. 227.2 min; P=0.000). No other significant differences were observed on any scale.
Table 1
| Characteristics | nCRT+ (n=51) | nCRT− (n=55) | P value |
|---|---|---|---|
| Gender, n (%) | 0.644 | ||
| Male | 31 (60.8) | 31 (56.4) | |
| Female | 20 (39.2) | 24 (43.6) | |
| Age, mean ± SD (years) | 55.9±10.1 | 56.2±10.7 | 0.892 |
| BMI, mean ± SD (kg/m2) | 25.0±3.0 | 24.6±3.1 | 0.445 |
| Tumour distance from the anal verge, mean ± SD (cm) | 4.0±1.6 | 4.3±1.7 | 0.411 |
| Tumor size, mean ± SD (cm) | 2.8±1.3 | 4.1±1.7 | 0.000 |
| Previous abdominal surgery, n (%) | 5 (9.8) | 8 (14.5) | 0.457 |
| ASA score, n (%) | 0.150* | ||
| I/II | 45 (88.2) | 53 (96.4) | |
| III/IV | 6 (11.8) | 2 (3.6) | |
| TMN status, n (%) | 0.083 | ||
| II | 29 (56.9) | 22 (40.0) | |
| III | 22 (43.1) | 33 (60.0) | |
| Type of operation, n (%) | 0.022* | ||
| Low anterior resection | 17 (33.3) | 31 (56.4) | |
| Abdominoperineal resection | 33 (64.7) | 22 (40.0) | |
| Hartmann’s procedure | 1 (2.0) | 2 (3.6) | |
| Operative procedure, n (%) | |||
| Open resection | 3 (5.9) | 7 (12.7) | |
| Laparoscopic surgery | 48 (94.1) | 48 (87.3) | |
| Type of LLND, n (%) | 0.124 | ||
| Unilateral | 33 (64.7) | 43 (78.2) | |
| Bilateral | 18 (35.3) | 12 (21.8) | |
| Blood loss, mean ± SD (mL) | 98.6±119.3 | 83.0±106.0 | 0.474 |
| Operation time, mean ± SD (min) | 296.6±72.0 | 227.2±80.0 | 0.000 |
| Hospital stay after operation, mean ± SD (days) | 8.1±6.0 | 8.4±4.8 | 0.764 |
| Postoperative complications1, n (%) | 0.648 | ||
| No | 36 (70.6) | 41 (74.5) | |
| Yes | 15 (29.4) | 14 (25.5) | |
| Circumferential resection margin status | 0.456 | ||
| R0 | 49 (96.1) | 51 (92.7) | |
| R1/2 | 2 (3.9) | 4 (7.3) |
1, exclude urogenital dysfunction; *, Fisher’s exact test. nCRT, neoadjuvant chemoradiotherapy; SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; LLND, lateral lymph node dissection.
Urinary function
The data on urinary function were summarized in Table 2. Although the mean IPSS score was higher in CRT+ group, there was no statistically significant difference (9.86 vs. 9.43; P=0.778) (Figure 2). The variations in the incidence of particular urinary function grade (P=0.143) were not significant. Similarly, although more common in the nCRT+ group than in the nCRT− group, the differences between the groups in terms of the incidence of urinary dysfunction (51.0% vs. 34.5%; P=0.087) were not significant.
Table 2
| Characteristics | nCRT+ (n=51) | nCRT− (n=55) | P value |
|---|---|---|---|
| Urinary function grade1, n (%) | 0.143 | ||
| Normal function | 25 (49.0) | 36 (65.5) | |
| Moderate dysfunction | 17 (33.3) | 15 (27.3) | |
| Severe dysfunction | 9 (17.6) | 4 (7.3) | |
| Postoperative urinary dysfunction2, n (%) | 26 (51.0) | 19 (34.5) | 0.087 |
| IPSS scores, mean ± SD | 9.86±7.26 | 9.43±8.18 | 0.778 |
1, 0–7 points: normal function, 8–19 points: moderate dysfunction, 20–35 points: severe dysfunction; 2, postoperative urinary dysfunction: the sum of moderate dysfunction and severe dysfunction. nCRT, neoadjuvant chemoradiotherapy; IPSS, International Prostatic Symptom Score; SD, standard deviation.
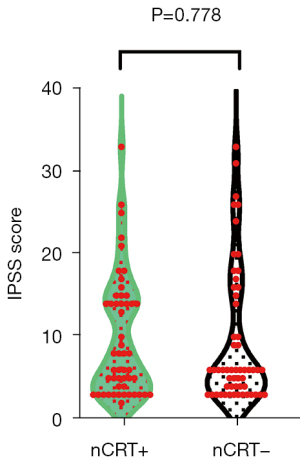
Table 3 summarizes univariable analyses of urinary function, which showed that patients’ age, sex, BMI, tumour size, ASA score, TNM status, neoadjuvant therapy, type of LLND, blood loss and operation time were not significantly associated with urinary disorder; tumour distance from the anal verge ≤4 cm (P=0.029) and type of operation (P=0.020) were significantly associated with urinary dysfunction. Multivariable analyses revealed that tumour distance from the anal verge was an independent risk factor for urinary dysfunction (OR =2.505; 95% CI: 1.080–5.813; P=0.032).
Table 3
| Characteristics | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| % of urinary dysfunction | P value | Wald | OR | 95% CI | P value | ||
| Gender | 0.503 | ||||||
| Male | 28 (45.2) | ||||||
| Female | 17 (38.6) | ||||||
| Age | 0.198 | ||||||
| ≥50 years | 36 (46.2) | ||||||
| <50 years | 9 (32.1) | ||||||
| BMI | 0.870 | ||||||
| ≥24 kg/m2 | 28 (43.1) | ||||||
| <24 kg/m2 | 17 (41.5) | ||||||
| Tumour distance from the anal verge | 0.029 | 4.547 | 2.505 | 1.080–5.813 | 0.032 | ||
| ≤4 cm | 12 (29.3) | ||||||
| >4 cm | 33 (50.8) | ||||||
| Tumour size | 0.556 | ||||||
| ≥3.5 cm | 24 (45.3) | ||||||
| <3.5 cm | 21 (39.6) | ||||||
| ASA score | 0.053 | 0.2172 | |||||
| I/II | 39 (39.8) | ||||||
| III/IV | 6 (75.0) | ||||||
| TNM status | 0.297 | ||||||
| II | 19 (37.3) | ||||||
| III | 26 (47.3) | ||||||
| Neoadjuvant therapy | 0.087 | 2.816 | 0.504 | 0.227–1.122 | 0.093 | ||
| Yes | 26 (51.0) | ||||||
| No | 19 (34.5) | ||||||
| Type of operation | 0.0201 | 0.2422 | |||||
| Low anterior resection | 14 (29.2) | ||||||
| Abdominoperineal resection | 30 (54.5) | ||||||
| Hartmann’s procedure | 1 (33.3) | ||||||
| Type of LLND | 0.581 | ||||||
| Unilateral | 31 (40.8) | ||||||
| Bilateral | 14 (46.7) | ||||||
| Blood loss | 0.165 | ||||||
| ≥50 mL | 30 (38.5) | ||||||
| <50 mL | 15 (53.6) | ||||||
| Operation time | 0.151 | ||||||
| ≥250 min | 27 (49.1) | ||||||
| <250 min | 18 (35.3) | ||||||
1, Fisher’s exact test; 2, multivariable analysis was analyzed by logistic regression (forward LR), these variables are not included in the equation. BMI, body mass index; ASA, American Society of Anesthesiologists; LLND, lateral lymph node dissection.
Male sexual function
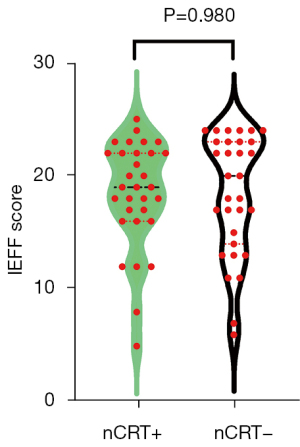
Meanwhile, female patients were excluded from the analyses of sexual dysfunction, leaving 31 patients in the nCRT+ and 31 patients in the nCRT− group for evaluation. The postoperative male sexual function of the two groups were quantified as previously described and summarised in Table 4. In addition to mean score of IEEF score, the difference of specific levels of erectile function was compared between the two groups. Total mean IIEF score was 18.45±4.72 (nCRT+) vs. 18.42±5.32 (nCRT−); P=0.980 (Figure 3), similarly to the IIEF score, the differences in terms of the incidence of each level of sexual (P=0.953) function and sexual dysfunction (P=0.607) were not significant.
Table 4
| Characteristics | nCRT+ (n=31) | nCRT− (n=31) | P value |
|---|---|---|---|
| Erectile function1, n (%) | 0.953 | ||
| Normal | 12 (38.7) | 14 (45.2) | |
| Mild | 12 (38.7) | 10 (32.3) | |
| Moderate | 5 (16.1) | 5 (16.1) | |
| Severe | 2 (6.5) | 2 (6.5) | |
| Postoperative sexual dysfunction2, n (%) | 19 (61.3) | 17 (54.8) | 0.607 |
| IIEF-5 scores, mean ± SD | 18.45±4.72 | 18.42±5.32 | 0.980 |
1, 21–25 points: normal erectile function, 16–20 points: mild erectile dysfunction, 11–15 points: moderate erectile dysfunction, 5–10 points: severe erectile dysfunction; 2, postoperative sexual dysfunction: the sum of mild, moderate and severe erectile dysfunction. nCRT, neoadjuvant chemoradiotherapy; IIEF, International Index Erectile Function; SD, standard deviation.
We considered that patient age (P=0.026) and type of LLND (P=0.044) were factors that might be associated with sexual dysfunction (Table 5). Given the results, we concluded that older individuals (≥50 years) were more prone to suffer from sexual dysfunction (OR =3.654; 95% CI: 1.028–12.982; P=0.045).
Table 5
| Characteristics | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| % of sexual dysfunction | P value | Wald | OR | 95% CI | P value | ||
| Age | 0.026 | 4.013 | 3.654 | 1.028–12.982 | 0.045 | ||
| ≥50 years | 31 (66.0) | ||||||
| <50 years | 5 (33.3) | ||||||
| BMI | 0.189 | ||||||
| ≥24 kg/m2 | 22 (52.3) | ||||||
| <24 kg/m2 | 14 (70.0) | ||||||
| Tumour distance from the anal verge | 0.165 | ||||||
| ≤4 cm | 13 (48.1) | ||||||
| >4 cm | 23 (65.7) | ||||||
| Tumour size | 0.829 | ||||||
| <3.5 cm | 17 (56.7) | ||||||
| ≥3.5 cm | 19 (59.4) | ||||||
| ASA score | 0.674 | ||||||
| I/II | 33 (58.9) | ||||||
| III/IV | 3 (50.0) | ||||||
| TNM status | 0.934 | ||||||
| II | 17 (58.6) | ||||||
| III | 19 (57.6) | ||||||
| Neoadjuvant therapy | 0.607 | ||||||
| Yes | 19 (61.3) | ||||||
| No | 17 (54.8) | ||||||
| Type of operation | 0.106 | 1.938 | 0.455 | 0.150–1.379 | 0.164 | ||
| Low anterior resection | 12 (46.2) | ||||||
| Abdominoperineal resection | 24 (66.7) | ||||||
| Type of LLND | 0.044 | 2.842 | 3.100 | 0.832–12.555 | 0.092 | ||
| Unilateral | 22 (50.0) | ||||||
| Bilateral | 14 (77.8) | ||||||
| Blood loss | 0.501 | ||||||
| ≥50 mL | 28 (56.0) | ||||||
| <50 mL | 8 (66.7) | ||||||
| Operation time | 0.436 | ||||||
| ≥250 min | 20 (54.1) | ||||||
| <250 min | 16 (64.0) | ||||||
BMI, body mass index; ASA, American Society of Anesthesiologists; LLND, lateral lymph node dissection.
Discussion
CRC is the fourth most common malignant tumour and the second leading cause of cancer-related death in the world (1). Advances in surgical procedures have decreased local recurrence rates and improved long-term survival, but surgeons are faced with yet another issue. Postoperative urogenital dysfunction imposes a heavy burden on rectal cancer patients at present. However, oncological outcomes should not be pursued at the cost of patient quality of life (20). Urogenital function largely lies in the integrity of the pelvic autonomic nerve, and avoid damage to this area is of great significance to the recovery of the patient’s urogenital function after surgery (20,21). Several studies have revealed that LLND increased the incidence of urogenital disorder because of neurological damage (15-17). In addition, previous studies reported that radiotherapy might lead to fibrosis in the nerve sheath, resulting in secondary demyelination (18); radiotherapy could also damage the capillaries of the neurovascular bundles, resulting in vascular impotence (22). After nCRT, oedema and fibrotic alterations in the tissue make dissection more difficult, resulting in neurological injury by accident. To date, some trials have indicated that surgery is the main factor related to postoperative urogenital dysfunction (23,24). However, there is little consensus as to whether nCRT would be detrimental to urogenital function in rectal cancer patients undergoing LLND. Our study aimed to investigate the extra adverse effect of nCRT + TME + LLND on urogenital function and explore the candidate risk factors.
An international multicentre trial involving 1,861 patients with rectal cancer proved that preoperative radiotherapy was not associated with urinary incontinence (38.7% vs. 37.6%; P=0.815) and difficulties in bladder emptying (27.3% vs. 37.8%; P=0.136) (23). Similarly, an analysis of the Surgical Department of the University of Heidelberg by Contin et al. (25) revealed that nCRT was not associated with urinary incontinence (preoperative short-term radiotherapy: 55.1% vs. 44.9%; chemoradiotherapy: 47.2% vs. 52.8%; P=0.66) and sexual dysfunction (preoperative short-term radiotherapy: 72.4% vs. 27.6%; chemoradiotherapy: 76.7% vs. 24.3%; P=0.27). In our cohort, 106 patients were included in the assessment of urinary dysfunction, and 62 male patients were included in the evaluation of sexual dysfunction. The two groups had comparable incidence of sexual dysfunction (61.3% vs. 54.8%) and urinary dysfunction (51.0% vs. 34.5%). The results showed that both the nCRT+ group and the nCRT− group suffered from sexual dysfunction and urinary dysfunction, and the intergroup difference in the occurrence was not prominent (P=0.607 and P=0.087). Due to differences in diagnostic standard and treatment options, the frequency of urogenital disorders in our cohort was not exactly consistent with the previous study. However, we argued that the cause of postoperative urogenital dysfunction was largely due to surgery rather than nCRT based on the above analysis.
In addition, we discovered that the operation period was significantly prolonged in the nCRT+ group (P=0.000). As already alluded, nCRT not only triggered fibrosis in sphincters but also damaged the neurovascular bundle of capillaries (18,19,22). We considered that the above factors might add to the difficulty of surgery, which slowed the operation. However, the incidence of postoperative complications between groups was equal (29.4% vs. 25.5%, P=0.648). To date, nCRT, with a low recurrence rate and an optimal prognosis, has been defined as the standard treatment for rectal cancer around the world (26). Although nCRT would prolong the operation period, the occurrence of postoperative complications and urogenital disorders were not promoted; thus, nCRT could be regarded as a secure and workable treatment.
Akasu et al. (15)proposed that the extent of urogenital dysfunction depended not only on the extent of autonomic nerve resection in a large part but also on the extent of LLND. There is no consensus on the exact mechanism of LLND-induced genitourinary dysfunction, mechanical damage to nerve fibres and ischaemic damage caused by dissection of blood vessels (15). With regard to the risk of sexual disorder, univariable analyses summarized that bilateral lymph node dissection had a significantly higher incidence (P=0.044), which was in accordance with Akasu’s statement. Multivariate analyses demonstrated that sexual dysfunction was linked to age (P=0.045). Contin et al. (25) investigated sexual dysfunction after surgery in rectal cancer and reported that male sexual function was associated with age (OR =1.055; 95% CI: 1.01–1.10; P<0.01). JCOG0212 was the largest study that explored urinary disorder of LLND, which revealed that age was the only independent risk factor for male sexual disorder (P=0.02) (27). Our conclusion showed that age was an independent predictive factor of sexual disorder, which was in accordance with these studies. Similar to our research, female patients preferred to drop out rather than answer questions about sexual dysfunction, so female sexual dysfunction was not evaluated in the above studies.
Ito et al. (28) demonstrated that LAR tended to have a lower incidence of urinary disorder than APR (P=0.06), and a tumour centre located below the peritoneum (P=0.01) was significantly linked to an increased incidence of urinary disorder. Similarly, APR showed a significantly higher incidence of urinary dysfunction in the present study (P=0.020), we suggested that surgery below the peritoneal reflex plane was more likely to damage the autonomic nerves. Tumour distance from the anal verge (≤4 cm) was the only risk factor for postoperative urinary dysfunction (P=0.029) in our study, which was consistent with Benoist’s results (P<0.05) (29). Different surgery regimens might be adopted on the basis of the location of the tumour, and patients with ultra-low rectal cancer usually underwent APR in this cohort. As discussed above, APR led to a higher possibility of neurological damage than other surgical procedures, and tumour distance from the anal verge (≤4 cm) could increase the incidence of postoperative urinary dysfunction.
This study had the following limitations. First, this was a small sample size and a single-centre retrospective study, further multicentre prospective randomized trials are needed to confirm the above conclusions. Second, the two groups had potential differences in tumor volume, which might lead to selection bias. In addition, female sexual disorder has been relatively neglected as in previous studies due largely to their unwillingness to answer issues about sexuality, as well as 12 patients were lost to follow up because of reluctance to volunteer information about problems related to urogenital dysfunction. Thus, further large-scale and comprehensive studies are required to verify our conclusions.
Conclusions
In conclusion, this 28-month follow-up study found that nCRT combined with TME + LLND did not result in a significantly higher rate of urogenital dysfunction than TME + LLND alone. In addition, the distance between the tumor and the anal margin was linked to the occurrence of urinary failure, and age was an independent predictive factor of male sexual dysfunction.
Acknowledgments
Funding: This work was supported by the Capital’s Funds for Health Improvement and Research (No. 2016-2-4022).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-87/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-87/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-87/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-87/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Akiyoshi T, Watanabe T, Miyata S, et al. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg 2012;255:1129-34. [Crossref] [PubMed]
- Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018;23:1-34. [Crossref] [PubMed]
- Monson JR, Weiser MR, Buie WD, et al. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum 2013;56:535-50. [Crossref] [PubMed]
- Georgiou P, Tan E, Gouvas N, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol 2009;10:1053-62. [Crossref] [PubMed]
- van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer 2014;50:1.e1-1.e34. [Crossref] [PubMed]
- Oh HK, Kang SB, Lee SM, et al. Neoadjuvant chemoradiotherapy affects the indications for lateral pelvic node dissection in mid/low rectal cancer with clinically suspected lateral node involvement: a multicenter retrospective cohort study. Ann Surg Oncol 2014;21:2280-7. [Crossref] [PubMed]
- Kim MJ, Hur BY, Lee ES, et al. Prediction of lateral pelvic lymph node metastasis in patients with locally advanced rectal cancer with preoperative chemoradiotherapy: Focus on MR imaging findings. PLoS One 2018;13:e0195815. [Crossref] [PubMed]
- Akiyoshi T, Matsueda K, Hiratsuka M, et al. Indications for Lateral Pelvic Lymph Node Dissection Based on Magnetic Resonance Imaging Before and After Preoperative Chemoradiotherapy in Patients with Advanced Low-Rectal Cancer. Ann Surg Oncol 2015;22:S614-20. [Crossref] [PubMed]
- Kim MJ, Chan Park S, Kim TH, et al. Is lateral pelvic node dissection necessary after preoperative chemoradiotherapy for rectal cancer patients with initially suspected lateral pelvic node? Surgery 2016;160:366-76. [Crossref] [PubMed]
- Wang P, Zhou S, Zhou H, et al. Evaluating predictive factors for determining the presence of lateral pelvic node metastasis in rectal cancer patients following neoadjuvant chemoradiotherapy. Colorectal Dis 2019;21:791-6. [Crossref] [PubMed]
- Song JH, Jeong JU, Lee JH, et al. Preoperative chemoradiotherapy versus postoperative chemoradiotherapy for stage II-III resectable rectal cancer: a meta-analysis of randomized controlled trials. Radiat Oncol J 2017;35:198-207. [Crossref] [PubMed]
- Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg 2017;266:201-7. [Crossref] [PubMed]
- Akasu T, Sugihara K, Moriya Y. Male urinary and sexual functions after mesorectal excision alone or in combination with extended lateral pelvic lymph node dissection for rectal cancer. Ann Surg Oncol 2009;16:2779-86. [Crossref] [PubMed]
- Shirouzu K, Ogata Y, Araki Y. Oncologic and functional results of total mesorectal excision and autonomic nerve-preserving operation for advanced lower rectal cancer. Dis Colon Rectum 2004;47:1442-7. [Crossref] [PubMed]
- Kyo K, Sameshima S, Takahashi M, et al. Impact of autonomic nerve preservation and lateral node dissection on male urogenital function after total mesorectal excision for lower rectal cancer. World J Surg 2006;30:1014-9. [Crossref] [PubMed]
- Bonnel C, Parc YR, Pocard M, et al. Effects of preoperative radiotherapy for primary resectable rectal adenocarcinoma on male sexual and urinary function. Dis Colon Rectum 2002;45:934-9. [Crossref] [PubMed]
- Heriot AG, Tekkis PP, Fazio VW, et al. Adjuvant radiotherapy is associated with increased sexual dysfunction in male patients undergoing resection for rectal cancer: a predictive model. Ann Surg 2005;242:502-10; discussion 510-1. [Crossref] [PubMed]
- Eveno C, Lamblin A, Mariette C, et al. Sexual and urinary dysfunction after proctectomy for rectal cancer. J Visc Surg 2010;147:e21-30. [Crossref] [PubMed]
- Lee DK, Jo MK, Song K, et al. Voiding and sexual function after autonomic-nerve-preserving surgery for rectal cancer in disease-free male patients. Korean J Urol 2010;51:858-62. [Crossref] [PubMed]
- Hall SJ, Basile G, Bertero EB, et al. Extensive corporeal fibrosis after penile irradiation. J Urol 1995;153:372-7. [Crossref] [PubMed]
- Lange MM, Maas CP, Marijnen CA, et al. Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br J Surg 2008;95:1020-8. [Crossref] [PubMed]
- Hendren SK, O'Connor BI, Liu M, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg 2005;242:212-23. [Crossref] [PubMed]
- Contin P, Kulu Y, Bruckner T, et al. Comparative analysis of late functional outcome following preoperative radiation therapy or chemoradiotherapy and surgery or surgery alone in rectal cancer. Int J Colorectal Dis 2014;29:165-75. [Crossref] [PubMed]
- Benson AB, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:874-901. [Crossref] [PubMed]
- Saito S, Fujita S, Mizusawa J, et al. Male sexual dysfunction after rectal cancer surgery: Results of a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for patients with lower rectal cancer: Japan Clinical Oncology Group Study JCOG0212. Eur J Surg Oncol 2016;42:1851-8. [Crossref] [PubMed]
- Ito M, Kobayashi A, Fujita S, et al. Urinary dysfunction after rectal cancer surgery: Results from a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for clinical stage II or III lower rectal cancer (Japan Clinical Oncology Group Study, JCOG0212). Eur J Surg Oncol 2018;44:463-8. [Crossref] [PubMed]
- Benoist S, Panis Y, Denet C, et al. Optimal duration of urinary drainage after rectal resection: a randomized controlled trial. Surgery 1999;125:135-41. [Crossref] [PubMed]


