
Diagnostic value of combined detection of CA72-4, CA19-9, and carcinoembryonic antigen comparing to CA72-4 alone in gastric cancer: a systematic review and meta-analysis
Introduction
Gastric cancer is a malignant tumor arising from the gastric mucosal epithelium and is one of the most common malignant tumors in humans. The vast majority of gastric cancers are adenocarcinomas, with no obvious symptoms in the early stage, or non-specific symptoms such as epigastric discomfort and belching, which are often similar to the symptoms of chronic gastric diseases such as gastritis and gastric ulcer and are easily ignored. Most patients have entered the middle and advanced stage at the time of diagnosis, and the prognosis is poor (1). Clinically, space-occupying lesions are often found by X-ray double contrast barium enema or endoscopy, or confirmed by biopsy or cytology (2). However, endoscopy is an invasive test that takes a long time and often causes significant discomfort in patients (3). Gastric histopathological examination is the gold standard for the diagnosis of gastric cancer, but it is invasive and not suitable for population screening (4). Carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, and CA72-4 are all serum tumor markers which applied in the diagnosis of pancreatic cancer and lung cancer (5). However, these 3 tumor markers are not specific for gastric cancer, their application value in patients with gastric cancer is still unclear. A previous study by Huang et al. (6) has shown that the method of combined detection can increase the accuracy of gastric cancer diagnosis to 66.0%. However, the results of such reports are varied, another study by He et al. (7) concluded that the sensitivity of CEA, CA125 and CA19-9 in the diagnosis of gastric cancer was 4.7–20.8% individually, and increased to 40.3% in combination. In this study, the diagnostic value of combined markers and individual markers was compared and evaluated by comprehensive quantitative meta-analysis to provide a basis for the diagnosis of gastric cancer. We present the following article in accordance with the PRISMA-DTA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-537/rc).
Methods
Inclusion criteria of the studies
We designed the inclusion criteria according to the Participants, Intervention, Control, Outcomes, Study (PICOS) principles: (I) study type: all the included studies were diagnostic studies, and were not limited in terms of whether there was only one center in the study. Studies could be prospective or retrospective studies, and both Chinese and English language studies were included; (II) participants: for prospective studies, patients were diagnosed with gastric cancer or non-gastric cancer (patients with gastric ulcer, gastritis, and gastric polyps, or healthy people in general) by “gold standard” (reference standard) before enrollment. For retrospective studies, patients were not divided into groups; (III) interventions & controls: fasting venous blood samples were taken from all participants in the morning, serum was separated after centrifugation, and CA72-4, CA19-9, and CEA concentrations in serum were measured using kits. The design of the diagnostic test should be in parallel; (IV) outcomes: data from combined testing of CA72-4, CA19-9, and CEA was required to be retrievable in the study. The reference standards included in the studies were different, but the diagnosis was mostly confirmed by gastroscopy and pathological section diagnosis.
Exclusion criteria of the studies
(I) Control studies, case series, reviews, experience sharing, case reports, and conference proceedings were excluded; (II) studies including animal serum were excluded; (III) studies that did not describe the reference standard for the diagnosis of gastric cancer with the purpose of predicting the prognosis of gastric cancer (diagnosis of non-gastric cancer) were excluded; (IV) studies that included 2 serum markers and more than 3 serum markers for the diagnosis of gastric cancer, but did not include the 3 serum markers CA72-4, CA19-9, and CEA described in this study were excluded; (V) data required for diagnostic meta-analysis could not be provided. The data extracted from each study included the number of true positive (TP) cases, false positive (FP) cases, false negative (FN) cases, and true negative (TN) cases. If the study could not provide this set of data, it would be excluded.
Literature search
We performed electronic searches by means of combined keyword searches, and the search keywords used were: “CA72-4”, “CA19-9”, “CEA”, “diagnosis”, and “gastric cancer”. PubMed, Embase, The Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang data were searched, and we limited the search time range from the time of database construction to December 2021.
Selection of studies
Two researchers independently completed the searches, removed duplicate documents, read the titles, abstracts, and full texts of the literature by using the de-duplication function of the Endnote X9 software, and excluded unqualified literature. In case of any dispute in this process, a third person could intervene and coordinate decisions after discussion.
Data extraction and conversion
After completing the screening of the literature, 2 researchers read the full texts of the literature again. The characteristic information of the studies (author, publication time, study site), information of study subjects (gender, age), diagnostic information (reference standard, sample size, diagnostic tools and process, diagnostic interval time), and diagnostic data (number of TP cases, FP cases, FN cases, TN cases) were extracted. If the diagnostic data could not be obtained from the study, we tried to calculate the TP, FP, FN, and TN data using the total number of patients, number of positive cases, number of negative cases, sensitivity, and specificity provided in the study.
Literature risk of bias assessment
We used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) scoring scale (8) to score the included diagnostic tests, which contains 14 evaluation contents, with 1 point for each evaluation. We only used 11 of them for evaluation (items 3, 8, and 9 were not necessary contents). The total score was 11 points, and more than 7 points (8) indicated better quality.
Statistical methods
Stata 16.0 (released by StataCorp LLC, Texas 77845, USA) was used for statistical analysis to calculate the pooled sensitivity (SENS), specificity (SPEC), and 95% confidence interval (CI). Heterogeneity was analyzed by the Q test. P<0.1 or I2>50% indicated significant heterogeneity, and a random-effects model was selected. A summary receiver operating characteristic (SROC) curve was drawn and the area under the ROC curve (AUC) was calculated, the Z-test was used to analyze whether there were differences in the diagnostic efficacy between the two, and when there was a threshold effect, the SROC curve showed a “shoulder-arm” distribution, otherwise there was no threshold effect. The evaluation of publication bias was conducted with Deek financial plot. If P>0.05, there was no publication bias in the included studies.
Results
Literature screening process and results
The literature retrieval flow chart is shown in Figure 1, and 535 articles were initially searched. After screening, a total of 10 articles (9-18) were included and selected, and a total of 6,574 patients participated in diagnosis, 3,077 for confirmed GC and 3,497 for non-GC respectively.
Basic characteristics of the studies
The basic characteristics of the included studies and patient characteristics are shown in Table 1. The diagnosis results are shown in Table 2. The minimum number of patients participating in diagnosis was 52, and the maximum was 3,534.
Table 1
| Author | Year | Sex ratio (M/F) | Age (years) | Number of subjects | Confirmed/ non-confirmed |
QUADAS score |
|---|---|---|---|---|---|---|
| Liang Y et al. (9) | 2016 | 2,377/1,157 | 55.83±11.98 | 3,534 | 1,945/1,589 | 8 |
| Ychou M et al. (10) | 2000 | 33/19 | 54.24±12.65 | 52 | 37/15 | 7 |
| Gan JC et al. (11) | 2014 | 152/80 | 53.98±15.46 | 232 | 111/121 | 9 |
| Yang L et al. (12) | 2020 | 630/502 | 63.38±5.76 | 1,132 | 500/632 | 10 |
| Guo J et al. (13) | 2017 | 522/261 | 55 [30–70] | 783 | 500/283 | 9 |
| Gong X et al. (14) | 2020 | 247/159 | 58 [47–78] | 406 | 200/206 | 8 |
| Tocchi A et al. (15) | 1998 | 36/23 | 51.76±5.48 | 59 | 37/22 | 8 |
| Yu J et al. (16) | 2018 | 172/44 | 57.43±10.55 | 216 | 167/49 | 7 |
| Tong GW et al. (17) | 2016 | 53/27 | 55.7±7.9 | 80 | 40/40 | 7 |
| Wei B et al. (18) | 2021 | 47/33 | 54.2±13.3 | 80 | 40/40 | 8 |
M/F, male/female; QUADAS, the Quality Assessment of Diagnostic Accuracy Studies.
Table 2
| Author | Cut-off value | Combined | CA72-4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | TP | FP | FN | TN | |||
| Liang Y et al. (9) | CEA ≥5 ng/mL, CA19-9 ≥27 U/mL, CA72-4 ≥5.3 U/mL | 943 | 347 | 1,002 | 1,242 | 536 | 237 | 1,409 | 1,352 | |
| Ychou M et al. (10) | N/A | 34 | 3 | 3 | 12 | 25 | 2 | 22 | 13 | |
| Gan JC et al. (11) | N/A | 48 | 20 | 63 | 101 | 33 | 6 | 78 | 115 | |
| Yang L et al. (12) | CEA ≥5.2 ng/mL, CA19-9 ≥37.7 U/mL, CA72-4 ≥7.45 U/mL | 351 | 170 | 149 | 462 | 439 | 158 | 61 | 474 | |
| Guo J et al. (13) | CEA ≥2.48 ng/mL, CA19-9 ≥28.81 U/mL, CA72-4 ≥2.47 U/mL | 415 | 12 | 85 | 271 | 220 | 28 | 280 | 255 | |
| Gong X et al. (14) | N/A | 136 | 6 | 64 | 200 | 80 | 19 | 120 | 187 | |
| Tocchi A et al. (15) | N/A | 26 | 4 | 11 | 18 | 22 | 3 | 15 | 19 | |
| Yu J et al. (16) | N/A | 50 | 3 | 117 | 46 | 72 | 8 | 95 | 41 | |
| Tong GW et al. (17) | CEA ≥10 ng/mL, CA19-9 ≥35 U/mL, CA72-4 ≥8.2 U/mL | 23 | 3 | 17 | 37 | 31 | 4 | 9 | 36 | |
| Wei B et al. (18) | CEA ≥5 ng/mL, CA19-9 ≥23 U/mL, CA72-4 ≥6.9 U/mL | 25 | 5 | 15 | 35 | 37 | 15 | 3 | 25 | |
CA, carbohydrate antigen; CEA, carcinoembryonic antigen; TP, true positive; FP, false positive; FN, false negative; TN, true negative; N/A, not available.
Meta-analysis results
Forest plots of diagnosis
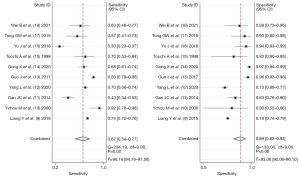
Ten articles included the combined diagnosis of CA72-4, CA19-9, and CEA, and there was heterogeneity between the articles (I2=96.16%, df=9.00, P=0.00). The diagnostic sensitivity obtained by random-effects model analysis was 0.67 (95% CI: 0.54, 0.77), the specificity was 0.89 (95% CI: 0.82, 0.93).
Ten articles included CA72-4 diagnosis alone, and there was heterogeneity between the articles (I2=98.84%, df=9.00, P=0.00). The diagnostic sensitivity obtained by random-effects model analysis was 0.58 (95% CI: 0.40, 0.73), the specificity was 0.86 (95% CI: 0.80, 0.90), as shown in Figures 2,3.
SROC curve
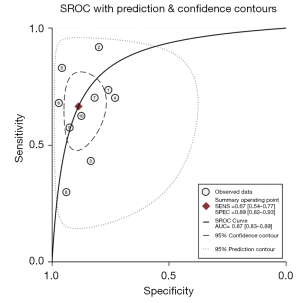
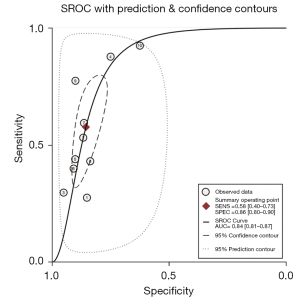
Figures 4,5 are SROC curves, and the AUC values of CA72-4, CA19-9, and CEA combined diagnosis and CA72-4 diagnosis alone were 0.87 (95% CI: 0.83, 0.89) and 0.84 (95% CI: 0.81, 0.87), respectively. The diagnostic efficacy comparison of combined detection and single detection of CA724 and was Z=4.86, P<0.05, the difference was statistically significant.
Source of heterogeneity
From the SROC curve, the curve did not present a “shoulder-arm”-like distribution, suggesting that there was no threshold effect.
Publication bias
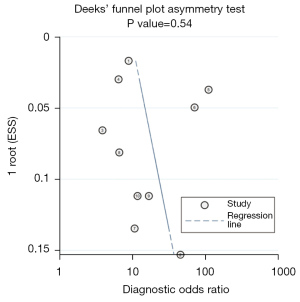
Figures 6,7 show the Deeks’ funnel plot asymmetry test, with P=0.54 and P=0.14 for combined diagnosis and single diagnosis respectively. Both P value >0.01 mean that there is no significant publication bias.

Discussion
Serum tumor markers are special biochemical substances released into the serum by tumor cells during differentiation and exist in the form of proteins, sugars, and enzymes. They can be used to detect the presence of tumors by their abnormal expression (19). Study (20) has shown that the serum concentrations of CEA, CA19-9, and CA72-4 in patients with gastric cancer increase to varying degrees, which is the basis for their use as tumor markers. The ideal tumor marker should have the characteristics of high sensitivity, high specificity, easy detection, prediction of cancer stage, and evaluation of prognosis. However, due to the complexity and polymorphism of the biological characteristics of tumor cells, there is still no single tumor marker that can meet all expectations. Several studies (20,21) have shown that the positive rate of CEA is in the range of 10.6–57.6%, CA19-9 is between 8.7–50.0%, and CA72-4 is between 18.6–58.0%. CA72-4 is mainly present in gastric, colon, pancreas, and other tumors, and it has been shown that CA72-4 has a higher SENS than other tumor markers in the diagnosis of gastric cancer and is a reliable indicator for the diagnosis of gastric cancer. However, CA72-4 alone has a lower SEN when used for the clinical diagnosis of gastric cancer (22). CA19-9 is produced by adenocarcinoma cells and is mainly found in tumors such as pancreatic cancer, gastric cancer, and colorectal cancer, especially in benign and malignant differentiation of digestive system tumors with great clinical significance (23). As a broad-spectrum tumor marker, CEA is an antigen common to tumor tissues and fetal cells, and some articles (23) have reported that CEA is significantly increased in colorectal cancer while being expressed at the lowest level in gastric cancer.
In this meta-analysis, 10 diagnostic studies were included, and a total of 6,574 patients participated in diagnosis. The results showed that the sensitivity and specificity of combined detection were 0.67 and 0.89, respectively. The sensitivity and specificity of CA72-4 detection alone were 0.58 and 0.86, respectively. The sensitivity and specificity of combined detection were higher than those of CA72-4 detection alone. Compared with CA72-4 detection alone, the DOR of combined detection was twice that of single detection (16 and 8, respectively), and the AUC of combined detection was higher than that of single detection (0.89 and 0.86, respectively), which indicated that combined detection had high discriminative ability and diagnostic efficiency for gastric cancer.
The 3 tumor markers CEA, CA19-9, and CA72-4 can be used not only as diagnostic indicators of gastric cancer, but also as predictors of gastric cancer stage. Studies have found that (24) that the positive rates of CEA, CA19-9, and CA72-4 expression in stage III–IV gastric cancer are higher than those in stage I-II gastric cancer, and CEA and CA72-4 are superior to CA19-9 in predicting the stage of gastric cancer, which also indicates that tumor markers have a close association with the pathological characteristics of gastric cancer. Study (25) suggests that the concentrations of CEA, CA19-9, and CA72-4 also have some reference for prognosis, and age >60 years, stage III, postoperative CEA elevation, and CA72-4 elevation are independent prognostic factors for gastric cancer recurrence.
This study still has some limitations as follows: (I) the number of included studies was small, and there was a lack of multi-center diagnostic tests with large sample sizes; (II) the main diagnostic purpose of some studies was to distinguish the stage and severity of gastric cancer, rather than the diagnosis of gastric cancer; (III) the included studies did not describe the reference standard; (IV) the diagnostic cut-off criteria of each study were not uniform, and some studies did not state the diagnostic cut-off value.
Conclusions
The combined use of CEA, CA19-9, and CA72-4 tumor markers in the diagnosis of gastric cancer has higher sensitivity and specificity than single marker diagnosis, and can be used for the screening of gastric cancer, as well as the auxiliary detection means for traditional imaging detection and histopathological examination.
Acknowledgments
Funding: The study was funded by
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-537/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-537/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol 2013;107:230-6. [Crossref] [PubMed]
- Hirasawa T, Aoyama K, Tanimoto T, et al. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer 2018;21:653-60. [Crossref] [PubMed]
- Yao K, Uedo N, Kamada T, et al. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc 2020;32:663-98. [Crossref] [PubMed]
- Rehena Z, Ghosh CK, Afroz F, et al. Comparison of Serum CA72-4 and CEA Levels in Patient with Endoscopically Suspected Gastric Carcinoma. Mymensingh Med J 2015;24:542-9. [PubMed]
- Yang AP, Liu J, Lei HY, et al. CA72-4 combined with CEA, CA125 and CAl9-9 improves the sensitivity for the early diagnosis of gastric cancer. Clin Chim Acta 2014;437:183-6. [Crossref] [PubMed]
- Huang C, Xiao L, Luo HL, et al. Preoperative neutrophil-to-lymphocyte ratio combined with serum CEA, CA19-9, CA125 and CA72-4 levels in the clinical pathological staging of gastric cancer-based on propensity score matching. J Biol Regul Homeost Agents 2020;34:1111-6. [PubMed]
- He CZ, Zhang KH, Li Q, et al. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol 2013;13:87. [Crossref] [PubMed]
- Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [Crossref] [PubMed]
- Liang Y, Wang W, Fang C, et al. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget 2016;7:49565-73. [Crossref] [PubMed]
- Ychou M, Duffour J, Kramar A, et al. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers 2000;16:105-10. [Crossref] [PubMed]
- Gan JC, Liu N, Wang DH, et al. Application value of serum tumor markers CEA, CA19-9 and CA72-4 in gastric cancer. Chinese General Medicine 2014;12:882-4.
- Yang L, Li XN, Yang XX. Value of combined detection of serum tumor markers CEA, CA19-9 and CA724 in the diagnosis of gastric cancer. Journal of Clinical and Experimental Medicine 2020;19:387-91.
- Guo J, Chen S, Li S, et al. A novel classifier based on three preoperative tumor markers predicting the cancer-specific survival of gastric cancer (CEA, CA19-9 and CA72-4). Oncotarget 2017;9:4814-22. [Crossref] [PubMed]
- Gong X, Zhang H. Diagnostic and prognostic values of anti-helicobacter pylori antibody combined with serum CA724, CA19-9, and CEA for young patients with early gastric cancer. J Clin Lab Anal 2020;34:e23268. [Crossref] [PubMed]
- Tocchi A, Costa G, Lepre L, et al. The role of serum and gastric juice levels of carcinoembryonic antigen, CA19.9 and CA72.4 in patients with gastric cancer. J Cancer Res Clin Oncol 1998;124:450-5. [Crossref] [PubMed]
- Yu J, Zheng W. An Alternative Method for Screening Gastric Cancer Based on Serum Levels of CEA, CA19-9, and CA72-4. J Gastrointest Cancer 2018;49:57-62. [Crossref] [PubMed]
- Tong GW, Huang WB. Diagnostic value of serum CEA, CA19-9 and CA724 in gastric cancer. Journal of Practical Clinical Medicine 2016;20:52-4.
- Wei B. Application value of CEA, CA724 and CA199 tumor markers in the diagnosis of gastric cancer. Clinical Medical Research and Practic 2021;6:116-8.
- Kim JH, Jun KH, Jung H, et al. Prognostic Value of Preoperative Serum Levels of Five Tumor Markers (Carcinoembryonic Antigen, CA19-9, Alpha-fetoprotein, CA72-4, and CA125) in Gastric Cancer. Hepatogastroenterology 2014;61:863-9. [PubMed]
- Sun Z, Zhang N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J Surg Oncol 2014;12:397. [Crossref] [PubMed]
- Shimada H, Noie T, Ohashi M, et al. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 2014;17:26-33. [Crossref] [PubMed]
- Jing JX, Wang Y, Xu XQ, et al. Tumor markers for diagnosis, monitoring of recurrence and prognosis in patients with upper gastrointestinal tract cancer. Asian Pac J Cancer Prev 2014;15:10267-72. [Crossref] [PubMed]
- Jiexian J, Xiaoqin X, Lili D, et al. Clinical assessment and prognostic evaluation of tumor markers in patients with gastric cancer. Int J Biol Markers 2013;28:192-200. [Crossref] [PubMed]
- Sisik A, Kaya M, Bas G, et al. CEA and CA 19-9 are still valuable markers for the prognosis of colorectal and gastric cancer patients. Asian Pac J Cancer Prev 2013;14:4289-94. [Crossref] [PubMed]
- Kim DH, Oh SJ, Oh CA, et al. The relationships between perioperative CEA, CA 19-9, and CA 72-4 and recurrence in gastric cancer patients after curative radical gastrectomy. J Surg Oncol 2011;104:585-91. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)




