
Analysis of DWI in the classification of glioma pathology and its therapeutic application in clinical surgery: a case-control study
Introduction
Glioma is the most common primary brain malignant tumor, accounting for more than 35% of all intracranial tumors (1). Clinically, glioma originates from the canceration of brain glial cells, and displays varied clinical characteristics such as low cure rate, high mortality rate, and high recurrence rate. It poses a severe threat to the physical and mental health of patients. Some previous studies have shown that the survival rate of patients with glioma is closely related to the stage of the tumor, thus accurate diagnosis and surgical resection are of great significance to the prognosis of patients (2-4). With the development and progress of magnetic resonance imaging (MRI) technology in recent years, MRI examination for brain tumor evaluation has also received increasing attention. The apparent diffusion coefficient (ADC) in magnetic resonance diffusion weighted imaging (DWI) can reflect the histological characteristics of gliomas and is a measurement and imaging technique for the diffusion motion of water molecules in vivo. Using the ADC values from the biopsy site provides better representation of the actual tumor pathology (5). This technology can be widely applied to classify gliomas and evaluate the extent of metastasis of glioma (3), which has significant value towards the preoperative evaluation of tumor lesions. Because traditional DWI ignores the effect of capillary microcirculation, it cannot accurately display the actual movement of water molecules. The ADC parameters derived from DWI are affected by many physiological factors, including cell volume fraction, the transverse relaxation rate, and membrane permeability, and their ability to reflect complex problems is not enough. In this study, we analyzed and assessed the clinical value and application of DWI in the pathological grading of glioma towards preoperative evaluation of tumors. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-114/rc).
Methods
General information
This study retrospectively analyzed the clinical data of 41 patients with glioma confirmed by surgery and pathology from January 1, 2019 to March 31, 2020 in the People’s Hospital of Gaozhou. The patients included 16 cases of low-grade gliomas and 25 cases of high-grade gliomas. Among the low-grade glioma patients, 11 were males and 5 were females, aged 5 to 80 years, with an average age of 41.26±2.01 years. High-grade glioma patients included 20 males and 5 females, aged 8 to 80 years old, with an average age of 42.04±2.15 years old. The general data of low-grade and high-grade gliomas were not significantly different, but were comparable to each other (P>0.05). Due to the limited sample size, this study did not meet the World Health Organization (WHO) classification standards and analysis of metabolites and ratios in the peritumoral edema area, which may still require more supporting evidence with future clinical practice. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of the People’s Hospital of Gaozhou (No. GYLLPJ-2021061). Written informed consent was obtained from the patients and their families.
Inclusion and exclusion criteria
Inclusion criteria
The inclusion criteria were as follows: (I) cases confirmed as glioma by surgical pathological examination; (II) cases did not receive any relevant treatment before DWI examination; (III) patients voluntarily accepted DWI examination; (IV) all patients and their families provided informed consent for this study.
Exclusion criteria
The exclusion criteria were as follows: (I) the patient’s cooperation in examination was extremely poor, and mental illness was present; (II) examination had serious DWI artifacts; (III) the tumor had severe necrosis; (IV) glioma was accompanied by other craniocerebral diseases; (V) patients with missing clinical data.
Study methods
DWI examination
Using a 3 T scanner and a 32-channel head coil, all patients were subjected to conventional MRI and DWI scans (axial SE-T1WI, T2-FLAIR, FSE-T2WI, DWI scans), followed by coronary, axial, sagittal, and coronal T1WI enhanced scanning. Scanning parameter settings were as follows: T1WI: TE 10 ms, TR 350 ms; T2WI: TE 90 ms, TR 3,500 ms; TLAIR: T1 1,000 ms; layer thickness set to 5 mm, layer spacing set to 1.5 mm, matrix set to 256×256, FOV(field of view) set to 24 cm × 24 cm. DWI scanning was performed using a single-shot SE-EPI diffusion sequence, and the imaging parameters were: TE 90 ms, TR 8,000 s, and matrix 128×128, while FOV, layer spacing, and layer thickness were the same as the above scan parameters. In order to reduce the influence of different factors on the measurement of DWI signals and values, the diffusion coefficient sensitive gradient field was applied to the phase encoding, layer selection X axis, Y axis, and Z axis directions. The diffusion sensitivity coefficient (b value) was 1,000 s/mm2, and the scan was maintained for 30 s. After the examination was completed, the contrast agent DTPA was injected through the elbow vein for enhanced scanning.
DWI image data processing
We used the Syngo workstation to perform image post-processing analysis and the ADC software and b value to establish the ADC map. Two experienced imaging doctors performed the ADC measurements on the glioma parenchyma, the peritumoral edema area, its relatively normal white matter area, and the contralateral normal white matter area by carefully avoiding the necrotic area, cystic changes, sulci, and ventricles. For measurement, each area was measured 3 times, and the average of the 3 values was taken as the final result.
Observation indicators
High-grade glioma and low-grade glioma imaging performance was analyzed with parameters such as shape and boundaries of the lesion, uneven signals, signs of necrosis, and the degree of cystic changes, followed by evaluation and comparison of the measured ADC values in each area.
Pathological examination
We selected some tumor tissues of patients for pathological analysis: after the tumor specimens were treated by paraffin fixation, sectioning and HE staining, we observed cell morphology and histological structure under light microscope, and conducted pathological grading according to WHO grading standards.
Statistical methods
The data was analyzed using Statistical Product and Service Solutions (SPSS) 22.0 software. Measurement data was expressed as mean ± standard deviation () and compared using the t-test. P<0.05 was considered statistically significant.
Results
Imaging performance of low-grade glioma
A total of 16 patients with low-grade gliomas showed relatively regular morphology (Figure 1). T2WI and T1WI showed high and low signals, respectively, with uniform signal expression and clear boundaries. There was no edema in the tumor, and the peritumoral edema range was narrower. DWI showed a slightly low signal or equal signal, and the enhanced scan of the tumor parenchyma had no or slight enhancement.
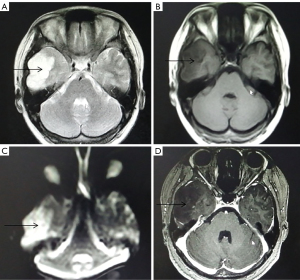
Imaging performance of high-grade glioma
A total of 25 patients with high-grade gliomas had irregular shapes, blurred boundaries, and uneven signals (Figure 2). T2WI and T1WI showed slightly higher and lower signals, respectively, with clear signs of necrosis. The degree of cystic changes was apparent, and some had bleeding. DWI of the parenchyma showed a slightly higher signal. The area of cystic changes and necrosis showed a lower signal. There was obvious peritumoral edema, with finger-like edema in 20 cases. However, on enhanced scans, the tumor parenchyma was obviously strengthened, and there were 17 cases which showed obvious ring-shaped enhancement (the ring-shaped enhancement was thick with uneven thickness).
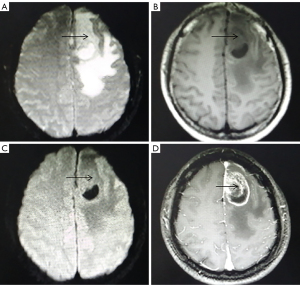
Comparison of ADC values in each observation area of low-grade and high-grade gliomas
The ADC values of low-grade gliomas in the tumor parenchyma, peritumoral edema, and white matter surrounding the edema area were significantly lower than those of high-grade gliomas, which was considered statistically significant (P<0.05). There was no significant difference between the two groups of patients with normal white matter ADC values on the contralateral side (P>0.05, Table 1).
Table 1
| Groups | Cases | Tumor parenchyma | Peritumoral edema | White matter surrounding edema area | Contralateral normal white matter |
|---|---|---|---|---|---|
| Low-grade glioma | 16 | 1.66±0.45 | 1.53±0.36 | 0.85±0.22 | 0.73±0.35 |
| High-grade glioma | 25 | 1.60±0.32 | 1.18±0.39 | 0.74±0.54 | 0.74±0.30 |
| T value | – | 21.058 | 20.226 | 18.762 | 0.582 |
| P value | – | 0.000 | 0.000 | 0.000 | 0.125 |
ADC, the apparent diffusion coefficient.
Discussion
Gliomas are also called neuroepithelial tumors as they occur in the neuroectoderm. The World Health Organization (WHO) classifies neuroepithelial tumors into astrocytomas, oligodendrogliomas, choroid plexus tumors, ependymomas, medulloblastomas, mixed tumors, and other neuroepithelial tumors. A major biological characteristic of gliomas is infiltrative growth, which cannot be easily distinguished from adjacent brain tissue. Especially for high-grade gliomas, complete resection is more difficult, and the postoperative recurrence rate is usually higher. The clinical treatment for glioma is surgical resection combined with radiotherapy and chemotherapy, of which surgical intervention is the preferred treatment for glioma. The principle of surgical intervention is to remove the tumor mass to the greatest possible extent under strict supervision with precautionary measures to ensure the complete restoration of normal functions. Therefore, accurate evaluation of the tumor grade before surgery is particularly critical for the implementation of accurate therapeutic strategies and the evaluation of tumor prognosis (4). The clinical diagnosis of gliomas mainly depends on conventional MRI examinations, which are mainly assessed through the clear identification of tumor signals, morphology, edema around the tumor, and the enhanced tumor augmentation, to initially differentiate and diagnose low-grade or high-grade gliomas. The edematous area which develops around the glioma is mainly angiogenic edema, and some edematous area are usually caused by tumor cell infiltration, but it is more challenging to differentiate them through conventional MRI examination. There are certain limitations in the accurate evaluation of infiltration which develops around the tumor (6). At present, DWI is the only non-invasive method for measuring the motion of the water molecules in living tissue. By measuring the ADC value, it can clarify the microscopic diffusion movement of water molecules inside and outside living tissues, and then understand the quantitative movement of water molecules (7). Choi et al. (8) reported that there is a close relationship between the ADC value and cell structure. The movement of water molecules will be hindered by the tumor cells, limiting their spread, and DWI examination can show these abnormal signals. The greater the density of tumor stroma, the smaller the extracellular space, and the free diffusion of water molecules is restricted, which may reduce the ADC value correspondingly. In contrast, when tumor density is lower, the diffusion capacity of water molecules increases, and the ADC value is enhanced.
DWI can quantitatively detect the permeability of immature tumor microvessels, mainly through the use of phase refocusing, to objectively and quantitatively evaluate the degree of microscopic diffusion of water molecules in tissues. DWI can efficiently evaluate the cell density, microvessel density, white matter cellulose integrity, and morphological changes of glioma tissue, and then effectively analyze the biological behavior of patients with gliomas (9). With the rapid advancement of technology, various MRI techniques have been applied to preoperative tumor grading of gliomas, especially in differentiating high-grade from low-grade gliomas. When the diffusion sensitivity coefficient (b value) of DWI imaging reaches 5,000 s/mm2 during the examination, the specific performance of the structural organization can be verified clearly. When the b value increases, the sensitivity for detecting water molecule diffusion can be improved, thereby reducing the T2 transmission effect. In recent years, more and more b values have been used in DWI examinations in clinical practice (10). The results of this study showed that the ADC value of the parenchyma of high-grade glioma was lower than that of low-grade glioma, which is consistent with the results of Togao et al. (11). Liu et al. (12) showed that the ADC value of the tumor parenchyma area had a sensitivity and specificity of 86.36% and 90.0%, respectively, for the identification of low and high-grade gliomas, indicating that the ADC value can more efficiently differentiate and grade gliomas. High-grade gliomas are more likely to infiltrate peritumoral cells. The smaller the distance from the tumor, the more cell infiltration will occur in the edema area. However, low-grade gliomas are less infiltrating into the surrounding cells (13).
The results of our study showed that the ADC values in the peritumoral edema and peripheral white matter areas of high-grade gliomas were lower than those of low-grade gliomas. In contrast, the ADC values of contralateral normal brain tissue were not significantly different. Moreover, it is apparent that the peritumoral edema ADC value can reveal the infiltration of high-grade glioma, and low-grade glioma has less obvious peritumoral infiltration. Therefore, DWI can also help clinicians judge the degree of peritumoral invasion of gliomas and effectively plan the surgical resection of glioma in a smooth and effective way. Existing studies have found that the DWI method alone is not specific and sensitive in the diagnosis of gliomas (14). Because traditional DWI ignores the effect of capillary microcirculation, it cannot accurately display the actual movement of water molecules. The ADC parameters derived from DWI are affected by many physiological factors, including cell volume fraction, the transverse relaxation rate, and membrane permeability, and their ability to reflect complex problems is relatively low. Moreover, due to the limited sample size, this study did not meet the WHO classification standards and analysis of metabolites and ratios in the peritumoral edema area, which may still require more supporting evidence with future clinical practice.
Federau et al. (15) have proposed an MRI method called intravoxel incoherent motion imaging, which can efficiently extract information on quantitative microvascular perfusion through multi-b-value DWI. The main parameters are pseudodiffusion coefficient (D*), perfusion fraction (f), the product of f and D* (f×D*), true diffusion coefficient (D), and ADC. Shen et al. found that the ADC and D values of high-grade gliomas were significantly lower than those of low-grade gliomas, and the f×D* values were significantly different in grades Ⅱ to Ⅳ gliomas (16). Recently, Togao et al. have reported that the f value of high-grade glioma is significantly higher than that of low-grade glioma (11). It can be seen that the 5 parameters derived from intravoxel incoherent motion imaging are promising markers for preoperative glioma grading, and the ADC value is only one of them. As our study reported here is based on single parameter analysis, more comprehensive studies designed with all 4 parameters can provide more conclusive evidence.
In summary, DWI has certain prognostic predictive value in the differential diagnosis of gliomas prior to surgical intervention, and can help clinicians plan a therapeutic strategy in a timely manner towards effective management of gliomas.
Acknowledgments
Funding: This work was supported by Maoming Science and Technology Project (Nos. 2018206, 2018216, 2020155) and Medical Scientific Research Foundation of Guangdong Province, China (No. B2021398).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-114/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-114/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-114/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of the People’s Hospital of Gaozhou (No. GYLLPJ-2021061). Written informed consent was obtained from the patients and their families.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bonm AV, Ritterbusch R, Throckmorton P, et al. Clinical Imaging for Diagnostic Challenges in the Management of Gliomas: A Review. J Neuroimaging 2020;30:139-45. [Crossref] [PubMed]
- Suh CH, Kim HS, Jung SC, et al. Diffusion-Weighted Imaging and Diffusion Tensor Imaging for Differentiating High-Grade Glioma from Solitary Brain Metastasis: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol 2018;39:1208-14. [Crossref] [PubMed]
- Rao PJ, Jyoti R, Mews PJ, et al. Preoperative magnetic resonance spectroscopy improves diagnostic accuracy in a series of neurosurgical dilemmas. Br J Neurosurg 2013;27:646-53. [Crossref] [PubMed]
- Shoaib Y, Nayil K, Makhdoomi R, et al. Role of Diffusion and Perfusion Magnetic Resonance Imaging in Predicting the Histopathological Grade of Gliomas - A Prospective Study. Asian J Neurosurg 2019;14:47-51. [Crossref] [PubMed]
- Glioma Imaging by O-(2-18F-Fluoroethyl)-L-Tyrosine PET and Diffusion-Weighted MRI and Correlation With Molecular Phenotypes, Validated by PET/MR-Guided Biopsies
- Liu J, Li C, Chen Y, et al. Diagnostic performance of multiparametric MRI in the evaluation of treatment response in glioma patients at 3T. J Magn Reson Imaging 2020;51:1154-61. [Crossref] [PubMed]
- Siddiqui H, Vakil S, Hassan M. Diagnostic Accuracy of Echo-planar Diffusion-weighted Imaging in the Diagnosis of Intra-cerebral Abscess by Taking Histopathological Findings as the Gold Standard. Cureus 2019;11:e4677. [Crossref] [PubMed]
- Choi HS, Kim AH, Ahn SS, et al. Glioma grading capability: comparisons among parameters from dynamic contrast-enhanced MRI and ADC value on DWI. Korean J Radiol 2013;14:487-92. [Crossref] [PubMed]
- Wang QP, Lei DQ, Yuan Y, et al. Accuracy of ADC derived from DWI for differentiating high-grade from low-grade gliomas: Systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e19254. [Crossref] [PubMed]
- Takayasu T, Yamasaki F, Akiyama Y, et al. Advantages of high b-value diffusion-weighted imaging for preoperative differential diagnosis between embryonal and ependymal tumors at 3 T MRI. Eur J Radiol 2018;101:136-43. [Crossref] [PubMed]
- Togao O, Hiwatashi A, Yamashita K, et al. Differentiation of high-grade and low-grade diffuse gliomas by intravoxel incoherent motion MR imaging. Neuro Oncol 2016;18:132-41. [Crossref] [PubMed]
- Liu ZL, Zhou Q, Zeng QS, et al. Noninvasive evaluation of cerebral glioma grade by using diffusion-weighted imaging-guided single-voxel proton magnetic resonance spectroscopy. J Int Med Res 2012;40:76-84. [Crossref] [PubMed]
- Horváth A, Perlaki G, Tóth A, et al. Increased diffusion in the normal appearing white matter of brain tumor patients: is this just tumor infiltration?. J Neurooncol 2016;127:83-90. [Crossref] [PubMed]
- Heo YJ, Kim HS, Park JE, et al. Uninterpretable Dynamic Susceptibility Contrast-Enhanced Perfusion MR Images in Patients with Post-Treatment Glioblastomas: Cross-Validation of Alternative Imaging Options. PLoS One 2015;10:e0136380. [Crossref] [PubMed]
- Federau C, Cerny M, Roux M, et al. IVIM perfusion fraction is prognostic for survival in brain glioma. Clin Neuroradiol 2017;27:485-92. [Crossref] [PubMed]
- Shen N, Zhao L, Jiang J, et al. Intravoxel incoherent motion diffusion-weighted imaging analysis of diffusion and microperfusion in grading gliomas and comparison with arterial spin labeling for evaluation of tumor perfusion. J Magn Reson Imaging 2016;44:620-32. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


