
The histologic effects of neoadjuvant stereotactic body radiation therapy (SBRT) followed by pulmonary metastasectomy—rationale and protocol design for the Post SBRT Pulmonary Metastasectomy (PSPM) trial
Introduction
Background and rationale
The use of non-surgical techniques for local control of primary and secondary lung tumors continues to garner increasing attention and adoption. The most widely researched non-surgical local modality as applies to lung malignancies is stereotactic body radiation therapy (SBRT) (1-3). While surgery remains the standard of care in the management of resectable primary and with pulmonary metastases, the prevalence of SBRT has increased, particularly in patients deemed to be non-operative candidates, and in those refusing to undergo surgery (4). The majority of research comparing surgery to SBRT has focused on post-treatment oncologic outcomes (survival and recurrence) in the context of non-small cell lung cancer (NSCLC). Using post-treatment imaging surveillance only, several trials have demonstrated 3-year local control rates of up to 90% (5). The use of radiographic surveillance post-SBRT is however limited by radiation induced lung injury leading to persistent radiologic abnormalities in the treatment field that can therefore misrepresent local control rates (6). There have been few efforts assessing the effectiveness of SBRT in tumor eradication at a histologic level. However, there were a few cases undergoing salvage surgery after SBRT for pulmonary metastases and viable cells were confirmed (7,8). In addition, little comparative research has evaluated SBRT vs. surgery in the treatment of pulmonary metastases for metastatic tumors deposited in the lung. In contrast, the role of pulmonary metastasectomy has been well researched and is supported by several retrospective reports demonstrating a survival advantage (9,10). In evaluating the histologic effect of SBRT on pulmonary metastasis, there may be rationale to consider this modality in select patients who would otherwise undergo surgery for disease that has already metastasized.
Objectives
For the local management of pulmonary malignancies, surgical resection and SBRT are considered as mutually exclusive treatment options. The primary objective of this study is to examine the effectiveness of SBRT on reducing tumor viability at a pathologic level, in hopes of concluding this information to both primary and pulmonary metastases. In surgical specimens post SBRT, rates of complete pathologic response (pCR) will be measured by a specialized lung pathologist using the International Association for the Study of Lung Cancer (IASLC) multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy (11). Also following secondary outcomes will be assessed: overall survival (OS) at 3 years, disease free survival (DFS) at 3 years, local recurrence rates, radiation related toxicity, postoperative pulmonary complication rate (including prolonged air leak, need for invasive or noninvasive mechanical ventilation, postop pneumonia and empyema), the effect on time-to-resection and tumour histology on pCR, and treatment effect on circulating tumor DNA (ctDNA) levels measured both after SBRT and surgery and compared to index level measured at the time of initial presentation.
Trial design
It is an open-label unblinded single-arm prospective trial evaluating induction SBRT followed by pulmonary metastasectomy. A phase 2 prospective trial which is a collaborative effort between Thoracic Surgery and Radiation Oncology to determine the effects of dual treatment of pulmonary metastasis amenable to curative resection with neoadjuvant SBRT followed by surgical resection, the Post SBRT Pulmonary Metastasectomy (PSPM) trial. We present this protocol in accordance with the SPIRIT reporting checklist (12) (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-232/rc).
Methods: participants, interventions, and outcomes
Study setting/participants eligibility criteria
This study will take place in an academic tertiary hospital, as a combined effort between two highly specialised divisions of Thoracic Surgery and Radiation Oncology. Recruitment will commence and continue until 39 patients are enrolled in the study via consecutive convenience sampling.
Patients inclusion criteria will be as follows: patient age ≥18 years; resectable pulmonary metastases without more effective systemic therapy option (regardless of type of primary malignancy, excluding hematologic malignancies) with the primary malignancy already having been treated without evidence of local recurrence; single-organ metastasis to lung only (except for colorectal cancer with synchronous hepatic metastasis); there should not be any evidence of nodal disease on pre-treatment CT scan; adequate pulmonary function to tolerate lung resection (post-operative predictive FEV1 ≥40% and DLCO ≥40%); and tumor(s) ≤5 cm. We set a number of no more than 5 metastases per lung based on restrictions from an SBRT standpoint. While it is true that number of mets is a prognostic indicator, data supports being able to completely resect these lesions as a more telling prognostic factor—hence our rational. Also, in consultation with our lung SBRT expert, we restricted treatment to lesions less than 5 cm in size—lesions with greater size would not be candidate for this trial and have traditionally been the upper limit for SBRT in other trials of oligometastatic disease. Patient having comorbidities not amenable to surgery; uncontrolled primary malignancy; hematologic malignancies (leukemia or lymphoma); >5 tumors in one lung; prior history of thoracic radiation; and history of lung cancer diagnosis within 5 years of assessment will be excluded.
Treatment & interventions
Preoperative intervention
Eligible patients will be initially evaluated by a thoracic surgeon. Consenting patients meeting inclusion criteria will be carefully evaluated for SBRT by the treating radiation oncologist with treatment commencing within 2 weeks, with planning and delivery parameters following the guidelines developed through the Canadian LUSTRE randomized SBRT lung trial for medically inoperable NSCLC (13). Specifically, SBRT will be delivered according to a risk-adjusted dose fractionation contingent on tumor size and location (48 Gy in 4 fractions for peripheral lesions, 60 Gy in 8 fractions for central or apical lesions). These doses are based on previously published data in the context of lung malignancies and are considered definitive SBRT doses given that we are evaluating the pCR of SBRT from a curative perspective. Following completion of SBRT, patients will be assessed at the 6-week mark by the treating radiation oncologist and thoracic surgeon with a post-treatment computed tomography (CT) of the chest.
Operative intervention
If no disease progression is identified, patients will undergo scheduled surgical resection of all metastatic tumors at 8–12 weeks post SBRT. The choice of lobar vs. sublobar resection will be determined by the participating surgeon, based on tumor size and location. Guidelines for pulmonary metastasectomy recommend lung-sparing surgical resection, with wedge resections being preferred if feasible. The majority of pulmonary metastasectomies are performed using minimally invasive (MIS) techniques via thoracoscopic or robotic assistance. The choice of surgical approach will be left to the discretion of the operating surgeon. In so doing, this methodology increases external validity. In the case of patients with bilateral metastatic nodules, SBRT will be administered to all sites of disease followed by staged resection of all malignant lesions. Only completely resectable tumors will be included in the study.
Clinical follow-up procedures
Patients will be seen post-SBRT and post-surgery between radiation and surgery 8 to 12 weeks and post-operative at 30 days for the initial postop visit, and then every 6 months for a total of 36 months after surgery with surveillance CT scans.
Outcome assessment
All patients will undergo radiographic and biochemical evaluation at baseline, post SBRT and postoperatively. The initial CT scan will be instrumental in determining patient eligibility and suitability to both SBRT and surgery. Surveillance imaging will be used to determine the initial and latent, incidence of local and distant recurrence, and to evaluate any associated post SBRT related parenchymal and mediastinal changes. These will be performed at 6 weeks post SBRT, and then at 6 months intervals post lung resection for a total of 3 years. In addition, we also specifically aim to explore whether cancer specific tumor biomarkers correlate with treatment effects at different treatment intervals (pre SBRT, post SBRT and postoperatively). For this purpose serum ctDNA levels will be measured using specialty assays at different time frames: baseline/pre-SBRT; 6 weeks post SBRT, and 6 weeks post-surgery. As small fragments of tumor DNA that usually comprise fewer than 200 building blocks (nucleotides) in length, the quantity ctDNA can help detect treatment effectiveness and prognostication.
Primary outcome
IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy will be used to assess the rates of complete pCR in surgical specimens post SBRT (11). The IASLC outlined the detailed recommendations on how to process lung cancer resection specimens and to define pCR, including major pCR or complete pCR after neoadjuvant therapy. A standardized approach was recommended to assess the percentages of viable tumor, necrosis, and stroma (including inflammation and fibrosis) (11). As such, a thoracic pathologist will perform the detailed pathologic review of surgical specimens according to these outlined criteria in order to assess the primary outcome of interest.
Secondary outcomes
Secondary outcomes to be assessed include: OS at 3 years, DFS and local recurrence rates at 3 years based on clinical and CT scan results, radiation-related toxicity evaluated using the RTOG Common Toxicity Criteria (14), 30-day postoperative pulmonary complication rate assessed using the Society of Thoracic Surgery (STS) post lung resection complication nomenclature, and the effect of treatment on ctDNA level.
Participant timeline
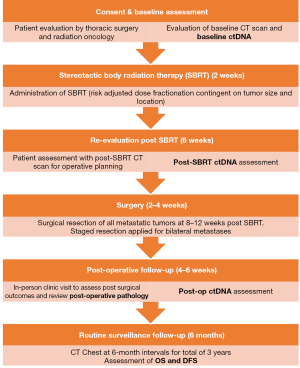
Figure 1 describes the time schedule of enrolment, interventions, assessments, and visits for participants. Briefly, eligible consenting patient will be evaluated for SBRT and receive treatment within 2 weeks of initial evaluation. Post-SBRT CT scan and ctDNA will be assessed at 6-week, with surgical resection planned at 8–12 weeks post SBRT. Following routine post-operative care, patients will have repeated levels of ctDNA drawn at 6-week postoperatively, and will undergo surveillance CT scans of the chest at 6-month intervals for a total of 3 years, or until disease recurrence is identified.
Sample size
The effect size estimate of pCR is the most important metric in determining the necessary sample size. The MISSILE study which evaluated SBRT prior to surgery in NSCLC, demonstrated a pCR of 60% (15). This is the only representative value available in the literature, even though the PSPM trial evaluates SBRT and surgery for metastatic disease and not primary lung cancer. On the other hand, the SBRT literature (using post-treatment CT scans only) reports local control rates of nearly 90%. The calculated sample size requirement is 39 patients, using the Fleming procedure, in order to measure the true pCR with a 95% confidence interval ± 10% using an estimated true pCR of 70%, estimated dropout rate of 20%, and 80% power.
Recruitment
From Institutional data, the Division of Thoracic Surgery at McMaster University performs an average of 450 pulmonary resections per year with approximately 10–15% being pulmonary metastasectomies for a spectrum of malignancies (including colorectal carcinoma, renal cell carcinoma and soft tissue sarcoma). It is estimated that the recruitment will be approximately 1–2 patients per month. In addition, the principal investigator is part of other disease-site multidisciplinary cancer conference groups and has presented this trial in order to promote collaboration and increase patient identification and recruitment. Regarding long-term follow-up, all patients undergoing pulmonary metastasectomies require cancer surveillance with dedicated CT scans of the chest. As such, there is no anticipation of any significant challenges to retention or significant loss to follow-up.
Data collection/analysis, management and monitoring
As a single-arm trial, all eligible study participants will undergo the same intervention and evaluation on a per-protocol basis. Subgroup analysis will be used to explore different prognostic related features associated with improved pCR post SBRT. Typical of Phase 2 trials, this is an unblinded study. Patients, investigators and outcome assessors will be aware of study protocol. It is important for radiologists and pathologists to know of prior SBRT in order to be able to assess radiographic and pCR rates on post treatment CT scans and in the final surgical specimen respectively. We do not believe that the unblinded nature of this Phase 2 trial will promote bias, given that there is no comparator group. In fact, treatment information may promote more detailed assessments by radiology and pathology.
Long-term patient data will be assessed as part of standard post malignant lung resection surveillance guidelines, given that all these patients undergo follow-up CT scan post resection to rule out disease recurrence. Survival data will be collected at the time of surveillance postop visits. Regarding descriptive statistics, continuous variables will be reported as means (standard deviation) and medians (range), while categorical data will be reported using rates and proportions. Primary and secondary outcomes will be analyzed using Fisher’s Exact test for categorical data and Student’s t-test for continuous data. Linear regression analysis will be used to assess differences in time to surgery post-SBRT on pCR rates. As part of the pre hoc secondary analysis, Cox-proportional hazard ratios will be used to evaluate OS and DFS. Survival analysis will be conducted using the log rank test.
The co-principal investigators of the trial will oversee all aspects of the study including management and training of study personnel, ensuring data integrity, overseeing data collection; assembly of a data-safety monitoring committee (DSMC), and analysis and dissemination of research findings. The DSMC will comprise a thoracic surgeon, radiation oncologist, molecular medicine specialist and statistician. The primary objective of the DSMC is to evaluate any significant adverse events related to treatment and ensure that the conduct of the trial does not pose increased risk to participants or lead to harm. The risks to participants in this study are pain, vomiting and weakness, which will be minimized and managed by continuous observation, appropriate medication by physicians. Interim analysis of the DSMC will take place once n=7 patients are treated per-protocol (8–12 months), concurrent with recruitment.
Ethics and dissemination
Current trial progress
This study has received local institutional approval from the local research ethics board, Hamilton Integrated Research Ethics Board (HiREB) (#7925; PSPM Trial Protocol v1.1 08May2020) and has successfully received funding by three local institutional grant committees. The study is registered with clinicaltrials.gov (NCT04160143). In addition, three additional Canadian centers have expressed interest in participation in hopes of rendering this a multicenter endeavor. Contract and data-sharing agreements have yet to be in place. Participant assessment and enrollment is soon to being in hopes of study completion by the end of study duration in 2 years.
We certify that this study will be conducted in accordance with the protocol and with the consensus ethical principles derived from international guidelines including the Declaration of Helsinki (as revised in 2013) and Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines, Applicable International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) Guidelines, and applicable laws and regulations. Patients or their legally acceptable representative must be consented. If allowable per local regulations, exceptions may be granted in cases where the patient is unable to provide informed consent.
Patients are typically evaluated preoperatively with several radiologic and functional tests to ensure that they meet surgical indications. After initial assessment, patients meeting inclusion criteria will be cross-referred for evaluation by a radiation oncologist to assess candidacy for SBRT. Eligible patients will then be approached (in-person or by phone) by a research assistant to consent to trial participation. The participants will have the option to withdraw at any point in time and will inform their respective treating physician and the study coordinator, who will notify the principal investigator. Participant baseline demographic information and outcome data will be recorded using Case Report Form (CRF) (Appendix 1) with data entered into REDCap, a web-based secure system that collects and stores the data behind secure institutional firewall. A tracking log of on-study patients will also be maintained on a secure institutional drive. The paper files and code will also be stored under lock-and-key. Participant data will be anonymized after follow-up is completed.
Given the set precedent and routine clinical use, we do not believe that the combination of SBRT and surgery would prove to be an ethical challenge. Most importantly, the combination treatment approach in the context of secondary metastases is distinctly different than primary malignancies, where delays in surgical resection may potentially compromise care. In the setting of pulmonary metastasectomies, the necessary wait time to surgery (as patients receive induction SBRT) actually provides several potential benefits: (I) induction radiation may increase the probability of negative resection margins (particularly in the context of multiple metastatic tumors), (II) demonstrating disease stability over 2–3 months (longer disease free-interval) with no new metastatic nodules validates surgical intervention and is an important prognostic factors, and (III) for patients undergoing chemotherapy, a lag period to surgical intervention may be necessary—receiving SBRT in the mean time would provide these patients with a form of treatment as they await surgery.
The study will be performed in accordance with the recommendations guiding physicians in biomedical research involving human subjects adopted by the 18th World Medical Assembly, Helsinki, Finland, 1964, amended at the 52nd World Medical Association General Assembly, Edinburgh, Scotland (October 2008). Informed written consent will be obtained from the patients prior to any study specific procedures. The right of a patient to refuse participation without giving reasons will be respected (Appendix 2). All members of the research team have received good clinical practice (GCP) training, honorary National Health Service (NHS) contracts, and adhere to NHS confidentiality guidelines and codes of conduct. In addition, this study has access to a number of private rooms in the outpatient and ward areas in which to consent patients and carry out the study processes.
Publication & dissemination policy
The project is registered with an authorised registry. The project’s success depends on the cooperation of all participants. For this reason, credit for the main results will be given to all those who have collaborated in the trial, through authorship and contribution. Uniform requirements for authorship for manuscripts submitted to medical journals will guide authorship decisions. So, the authorship credit should be based only on substantial contribution to: conception and design, acquisition of data, analysis, interpretation of data, drafting the article or revising it critically for important intellectual content, and/or final approval of the version to be published. Considering this, the principal investigator and relevant research staff, will be named as authors in any publication. Additionally, all collaborators will be listed as contributors for the main publication, giving details of roles in planning, conducting, and reporting. To maintain the scientific integrity of the project, data will not be released prior to the first publication of the analysis of the primary endpoint, either for publication or oral presentation purposes, without the permission of the research team and the DSMC. In addition, individual collaborators must not publish data concerning their participants which is directly relevant to the questions posed in the project until the first publication of the analysis of the primary endpoint.
Discussion & study impact
Much of the literature supporting pulmonary SBRT focuses on the treatment of NSCLC. The most widely reported study demonstrating the effectiveness of SBRT in the treatment of primary lung lesions is a pooled analysis of two randomized trials (STARS and ROSEL—both closed early due to poor recruitment). The unpowered analysis of 58 patients randomly assigned to lobectomy vs. SBRT demonstrated no difference in overall and recurrence-free survival at 3 years (OS: 79% vs. 95%, and 80% vs. 86%) (15). Surgery was associated with a 4% mortality rate and 44% high-grade complication rate. In contrast, 10% of SBRT patients had grade 3 adverse events, with no reported grade 4 events (15). This study is generally criticized for its lack of methodologic rigor and post hoc analysis. A recent meta-analysis of 19 trials spanning 2005–2018 of 1,434 patients demonstrated good local and regional control using SBRT, with similar complication rates to surgery. Three-year OS ranged from 43–95% with loco-reginal control rates as high as 98%. Up to 33% of patients, however, demonstrated distant recurrence at 3 years (1). This high distant recurrence rate could be secondary to low pCR as compared to complete surgical resection, an association that has yet to be evaluated.
Ibrahim et al. (16) evaluated the comparative effectiveness of surgery vs. other local control measures in the management of colorectal cancer pulmonary metastasis. The authors concluded that the highest level of evidence supported the use of surgery as the primary treatment modality. Although some patients underwent repeated surgical resection, no patients underwent combination treatment of surgery and SBRT. Generally, surgery was associated with low procedure-related mortality (0–2.4%) and an average 5-year OS rate of approximately 50%, while SBRT was associated with similar survival but only at 2-year (17). In addition, the surgical and non-surgical management of sarcoma pulmonary metastases can be more complex particularly with repeat thoracotomy (18), however given the relative lower incidence of sarcoma compared to other cancer subtypes, the effect of this on a Phase 2 trial is unlikely to be significant.Only one retrospective cohort study directly compared surgery vs. SBRT in colorectal cancer oligometastases, with most patients being offered surgery as first line treatment. The results demonstrated no difference in survival or local control comparing both modalities, but DFS was only 17% at 3 years in the entire cohort (19). Similarly, a recently conducted exploratory analysis demonstrated that OS after SBRT is similar to surgery for the first 2 years (20). Moreover, recent research has demonstrated the abscopal effect of SBRT, where radiation induced activation of the immune system can elicit immune-mediated anti-tumor responses (21). These results highlight the important principle that in the setting of pulmonary metastasis, distant recurrence remains a challenge. Despite the survival equivalence, patients tend to be treated surgically as the first option without addressing the risk of distant disease (22).
There only exists one trial evaluating the effects of combined modality pulmonary SBRT and surgery. The MISSILE trial evaluated the pCR rate of SBRT on primary lung cancer undergoing surgical resection for definitive cure. The pCR was 60% on histologic analysis, and the regional control rate was 56% at 2 years (17). The rationale behind this is unclear and may suggest worsening nodal spread of disease between the intervals of SBRT and surgical resection. This is less likely to be of concern in the setting of metastasectomy given that nodal disease control is not typically part of the management of pulmonary metastatic disease. It is possible that secondary malignancies represent a different local disease burden compared to primary lesions and accordingly a potentially different pCR following SBRT. This trial is therefore valuable in assessing the pCR in SBRT for metastatic disease, and in explaining the unexpectedly high regional recurrence rates of MISSILE.
Taken together, the literature supports the pursuit of this novel trial given the knowledge gaps that exist. The principles used in the management of primary vs. secondary lung malignancies differ, potentially creating greater equipoise between surgery and SBRT in the setting of metastatic disease. The SBRT plays a role as a potentially non-invasive treatment for small-volume tumors in the lung is well established, however the effectiveness of tumor eradication has yet to be determined (13,23,24). This trial (prospective Phase 2) will provide several novel contributions to the literature: (I) the SBRT effectiveness assessment in metastatic tumor control (radiotherapeutic metastasectomy), (II) by surgical resection, the evaluation of pCR to SBRT, (III) identification of histologic predictors of radiation effect and toxicity (i.e., what are the effects of SBRT on different type of metastatic disease), (IV) the effect of combined modality SBRT and surgery on survival and local recurrence as compared to either modality alone, and (V) assessing the association of SBRT and surgical resection on the levels of ctDNA and the burden of systemic disease at a biochemical level. This trial will exclusively evaluate whether surgery as an adjunct to radiotherapy offers better tumor control as compared to SBRT alone (compared to historical controls from previously published reports), and whether it decreases locoregional recurrence. By evaluating the pCR following SBRT, this study lays the groundwork for further research to evaluate if SBRT could be considered first line treatment in pulmonary metastasis. Most importantly, evaluation of pCR following SBRT is an important concept that has not been fully evaluated in the literature, and this trial will add to the results of the MISSILE study to determine the pCR of SBRT across different malignancies, and potentially identify prognostic features outlining which patients are best treated by SBRT and/or surgery.
In evaluating the effect of SBRT on pulmonary metastasis at a histologic level, this trial may increase the use of this modality in selected patients who would otherwise only undergo surgery for disease that has already metastasized. We believe this study to be innovative for several reasons. Firstly, to date there are limited published reports on the rates of pCR response following SBRT, with only one pilot study assessing this phenomenon in the setting of NSCLC. Secondly, this study will be able to accurately quantify the effects of SBRT on tumor eradication—and accordingly will provide a large contribution to the literature which is drastically lacking. Thirdly, no study has evaluated the role of induction SBRT in the treatment of pulmonary metastasis. Finally, SBRT may have a greater role in pulmonary metastases (as compared to primary lung cancer) given a theoretical lower burden of local disease, the fact that the majority of patients would have already received adjunctive chemotherapy, and that regional lymph node metastasis is less likely (with most disease spread being a consequence of a hematogenous phenomenon and not secondary to locoregional spread).
Moreover, at a local level, this study protocol has had the added impact improved communication and multidisciplinary care of patients with stage IV malignancies. Essentially, the promotion of the PSPM trial has facilitated multimodal care of patients and promoted the use of surgical intervention and radiotherapy in the treatment of patients with oligometastatic disease. As such, one of the unintended consequences has been the promotion of more aggressive local therapy for patients receiving chemotherapy only under the care or medical oncologists.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-232/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-232/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-232/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study has received local institutional approval from the local research ethics board, Hamilton Integrated Research Ethics Board (HiREB) (#7925; PSPM Trial Protocol v1.1 08May2020). The study is registered with clinicaltrials.gov (NCT04160143). We certify that this study will be conducted in accordance with the protocol and with the consensus ethical principles derived from international guidelines including the Declaration of Helsinki (as revised in 2013) and Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines, Applicable International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) Guidelines, and applicable laws and regulations. Patients or their legally acceptable representative must be consented. If allowable per local regulations, exceptions may be granted in cases where the patient is unable to provide informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Prezzano KM, Ma SJ, Hermann GM, et al. Stereotactic body radiation therapy for non-small cell lung cancer: A review. World J Clin Oncol 2019;10:14-27. [Crossref] [PubMed]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi89-98. [Crossref] [PubMed]
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Brada M, Pope A, Baumann M. SABR in NSCLC--the beginning of the end or the end of the beginning? Radiother Oncol 2015;114:135-7. [Crossref] [PubMed]
- Nguyen NP, Garland L, Welsh J, et al. Can stereotactic fractionated radiation therapy become the standard of care for early stage non-small cell lung carcinoma. Cancer Treat Rev 2008;34:719-27. [Crossref] [PubMed]
- Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)--can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012;102:335-42. [Crossref] [PubMed]
- Antonoff MB, Correa AM, Sepesi B, et al. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J Thorac Cardiovasc Surg 2017;154:689-99. [Crossref] [PubMed]
- Hamaji M, Mitsuyoshi T, Yoshizawa A, et al. Salvage Pulmonary Metastasectomy for Local Relapse After Stereotactic Body Radiotherapy. Ann Thorac Surg 2018;105:e165-8. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Pastorino U. The development of an international registry. J Thorac Oncol 2010;5:S196-7. [Crossref] [PubMed]
- Travis WD, Dacic S, Wistuba I, et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 2020;15:709-40. [Crossref] [PubMed]
- Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200-7. [Crossref] [PubMed]
- Swaminath A, Wierzbicki M, Parpia S, et al. Canadian Phase III Randomized Trial of Stereotactic Body Radiotherapy Versus Conventionally Hypofractionated Radiotherapy for Stage I, Medically Inoperable Non-Small-Cell Lung Cancer - Rationale and Protocol Design for the Ontario Clinical Oncology Group (OCOG)-LUSTRE Trial. Clin Lung Cancer 2017;18:250-4. [Crossref] [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. Erratum in: Lancet Oncol 2015;16:e427. [Crossref] [PubMed]
- Ibrahim T, Tselikas L, Yazbeck C, et al. Systemic Versus Local Therapies for Colorectal Cancer Pulmonary Metastasis: What to Choose and When? J Gastrointest Cancer 2016;47:223-31. [Crossref] [PubMed]
- Palma DA, Nguyen TK, Louie AV, et al. Measuring the Integration of Stereotactic Ablative Radiotherapy Plus Surgery for Early-Stage Non-Small Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019;5:681-8. [Crossref] [PubMed]
- Hamaji M, Chen F, Miyamoto E, et al. Surgical and non-surgical management of repeat pulmonary metastasis from sarcoma following first pulmonary metastasectomy. Surg Today 2016;46:1296-300. [Crossref] [PubMed]
- Widder J, Klinkenberg TJ, Ubbels JF, et al. Pulmonary oligometastases: metastasectomy or stereotactic ablative radiotherapy? Radiother Oncol 2013;107:409-13. [Crossref] [PubMed]
- Filippi AR, Guerrera F, Badellino S, et al. Exploratory Analysis on Overall Survival after Either Surgery or Stereotactic Radiotherapy for Lung Oligometastases from Colorectal Cancer. Clin Oncol (R Coll Radiol) 2016;28:505-12. [Crossref] [PubMed]
- Liu Y, Dong Y, Kong L, et al. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol 2018;11:104. [Crossref] [PubMed]
- Shultz DB, Filippi AR, Thariat J, et al. Stereotactic ablative radiotherapy for pulmonary oligometastases and oligometastatic lung cancer. J Thorac Oncol 2014;9:1426-33. [Crossref] [PubMed]
- Nuyttens JJ, van der Voort van Zyp NC, Praag J, et al. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiother Oncol 2012;102:383-7. [Crossref] [PubMed]
- Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest 2001;120:1584-91. [Crossref] [PubMed]


