
Overexpression of lncRNA MAPT-AS1 exacerbates cell proliferation and metastasis in breast cancer
Introduction
Breast cancer (BC) is one of the most common malignant tumors in women with the highest morbidity and mortality, seriously threatening the physical and mental health of females worldwide (1). An epidemiologic study reported that the 5-year survival of BC patients at the early stage is nearly 90%; however, the average 5-year survival in the advanced stage of BC is less than 40% (2). Although the rapid development of surgery, radiotherapy, chemotherapy, and other treatment approaches has improved the prognosis of BC patients, the clinical outcomes of most advanced stage patients remain unsatisfactory (3). Therefore, it is urgently required to elucidate the underlying mechanism of BC, which has great significance for seeking potential biotherapy targets.
Long non-coding RNAs (lncRNAs), which contain more than 200 nucleotides, have diverse features and functions such as remodeling chromatin and genome architecture, RNA stabilization, and transcription regulation, including enhancer-associated activity (4-6). Recently, studies have shown that multiple lncRNAs play crucial and interactive roles in the malignant progression of BC (7,8). For instance, it has been reported that the overexpression of lncRNA HOTAIR can promote growth, metastasis, and apoptosis of BC cells (9). The lncRNA GATA3-AS1 causes progressive and immunization of triple negative breast cancer (TNBC) by stabilizing programmed death-ligand 1 (PD-L1) protein and degrading Gata3 protein (10). However, their detailed roles in BC are still under scrutiny.
The lncRNA microtubule-associated protein tau-AS1 (MAPT-AS1) is located in Chr17q21.31. Recent study has shown that overexpression of MAPT-AS1 is closely related to the poor prognosis of BC patients, suggesting that MAPT-AS1 may be involved in the development of BC (11). Moreover, MAPT-AS1 overexpression may tie to the cell growth, invasiveness, and paclitaxel resistance in estrogen receptor (ER)-negative BC cells (12). In our previous study, 837 BC patients and 106 healthy cases from The Cancer Genome Atlas (TCGA) database were analyzed using RNA sequencing data, and we identified that MAPT-AS1 upregulation had the highest significance in the survival-related lncRNAs of BC (13). Online bioinformatics analysis showed that MAPT-AS1 has no coding capability (http://cpc.cbi.pku.edu.cn/programs/run_cpc.jsp). Till now, no study about the mechanism of MAPT-AS1 in BC has been reported, the role of MAPT-AS1 in cell proliferation and metastasis of BC remains unclear.
In this study, we aimed to detect the expression level of MAPT-AS1 in BC patients, and analyze the effect of MAPT-AS1 on the proliferation, migration, and invasion of BC cells, thus providing new insights for the clinical diagnosis and treatment biomarker for patients with BC. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-719/rc).
Methods
Sample collection
A total of 20 cases of BC and normal adjacent tissues were collected, and the serum samples from 498 BC patients, 222 patients with benign breast diseases, and 429 healthy controls were obtained from Shunde Hospital of Guangzhou University of Chinese Medicine (Guangzhou, China) from February 2019 to February 2020. The inclusion criteria for BC patients in our study were as follows: (I) postoperative histopathological diagnosis of BC; (II) complete information of medical records and pathological data before and after operation; (III) no radiotherapy or chemotherapy before operation; (IV) first BC resection. The exclusion criteria were as follows: (I) accompanied with other malignant tumors; (II) history of BC; (III) incomplete pathological data or uncertain diagnosis; (IV) patients who received preoperative chemoradiotherapy; (V) pregnant or lactating women. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committee of the Shunde Hospital of Guangzhou University of Chinese Medicine (No. KY-2020002) and informed consent was taken from all the patients. The tissue samples were impregnated with RNALater® (Product # AM7021, Thermo Fisher Scientific, Waltham, MA, USA) at 4 ℃ overnight, and stored at −80 ℃. Serum samples were stocked in RNase-free Eppendorf tubes at −80 ℃.
RNA extraction and quantitative reverse transcription-polymerase chain reaction
Total RNA from tissues and cell lines was extracted by TRIzol (Product #15596026, Invitrogen, Carlsbad, CA, USA), and Hipure blood RNA kits (Product #R4161-03, Maygen, Guangzhou, China) was used to extract RNA from serum samples. The quality of total RNA was assessed by NanoDrop 2000 spectrophotometer (Thermo Scientific, USA), for which the A260/A280 ratio was between 1.8–2.0. Complementary DNA (cDNA) was generated using 2 µL Prime ScriptTM RT Master Mix (Product #RR036A, Takara, Shiga, Japan), 0.5 µg total RNA, and up to 10 µL RNase-free water. Then, the samples were incubated at 37 ℃ for 15 min, 85 ℃ for 5 s, and the cDNA was stored at −80 ℃. Quantitative revrse transcription polymerase chain reaction (qRT-PCR) was completed using the QuantStudioTM 7 Flex Real-Time PCR System according to the manufacturer’s instructions of TB Green® Premix Ex TaqTM (Product #RR420A, Takara, Japan) under the following conditions: 95 ℃ for 30 s, followed by 45 cycles of 95 ℃ 15 s, and 60 ℃ for 1 min. Gene expression was normalized to 18S due to its stable feature in the serum. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control for tissues and cell lines samples. The sequences of MAPT-AS1 primers were as follows: 5'-CATGCTGGATTCTGAGCCACTCTC-3' (forward); 5'-TGAAGAAGCCTGGACAG GAGGTC-3' (reverse). The sequences of 18S primers were: 5'-GTAACCCGTTGAACCCCATT-3' (forward); 5'-CCATCCAATCGGTAGTAGCG-3' (reverse). The sequences of GAPDH primers were: 5'-CGGATTTGGTCGTATTGGG-3' (forward); 5'-CTGGAAGATGGTGATGGGATT-3' (reverse). The qRT-PCR reactions were performed in triplicate. The MAPT-AS1 relative expression levels were quantified using the 2−Δ∆Ct method.
Agarose gel electrophoresis
We made 3% gels with 50 mL 1 × Tris-borate-EDTA (TBE) and 1.5 g agarose, and the agarose/buffer mixture was melted by heating in the microwave oven. After cooling to below 60 ℃, 2 µL ethidium bromide (EB) was added, and the mixture was poured into the gel mold. After 20 min, the comb was removed and the gel was filled with 245 mL TBE buffer. A 5 µL aliquot of the PCR sample was mixed with 1 µL of loading buffer, and loaded in the ready gel. The parameters of electrophoresis were as follows: 20 V for 0.5 h, 40 V for 4 h. After electrophoresis, the gel was observed using a gel imaging system (bio-rad|Biorad,ChemiDoc XRS+).
Establishment of MAPT-AS1 knockdown or overexpression cell lines
The human normal breast epithelial cell line MCF-10A, and BC cell lines T47D, SKBR-3, ZR-75-1, MDA-MB-231, and MCF-7 (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, Carlsbad, CA, USA) including 10% fetal bovine serum (FBS; Gibco, USA) at 37 ℃ in 5% CO2.
The lentiviral MAPT-AS1-expression vector pCDH-MAPT-AS1 was constructed by cloning the human MAPT-AS1 gene intopCDH-CMV-MCS-EF1-copGFPvector (JiRan, Shanghai, China). We used MIT’s siRNA designer (http://sirna.wi.mit.edu/home.php) to design 4 quadruplexes targeting MAPT-AS1, and the most effective short hairpin RNA (shRNA) was screened for subsequent experiments. The corresponding sequence of the most effective one was as follows: 5'-GCTGGAAAGAGAACTCAAATTCAAGAGATTTGAGTTCTCTTTCCAGCTTTTTT-3'. The lentiviral particles were produced by the cotransfection of 293T cells with lentiviral packaging mix and a pLenti vector (psi-LVRH1GP-shMAPT-AS1, psi-LVRH1GP-shControl, pCDH-MAPT-AS1, or pCDH-NEO). Lentivirus-containing supernatant was harvested and infected MDA-MB-231 cells and ZR-75-1 cells. The stable cells were selected with puromycin at a concentration of 0.5 µg/mL (Product# A1113803, Thermo Fisher Scientific, USA) in the culture media.
Cell proliferation assay
The BC cells were inoculated in 96-well plates (100 µL/well; 3,000 cells/well), and incubated at 37 ℃ with 5% CO2. Then, 10 µL of cell counting kit-8 (CCK-8) solution (Product # CK04, Dojindo Co., Ltd., Tokyo, Japan) was added into each well at different time points, including 0, 24, 48, 72, and 96 h, then incubated for 3–4 h. The absorbance of each well was measured at 450 nm through a spectrophotometer reader. The experiment was performed in triplicate.
Colony formation assay
The BC cells were inoculated in 6-well plates (5,000 cells/well) and incubated at 37 ℃ with 5% CO2 for 14 days. The medium was changed every 2 days. The cells were fixed with 4% paraformaldehyde for 20 min and then stained with 0.1% crystal violet. The colonies with more than 50 cells were counted.
Migration and invasion assay
The established stable cell lines were seeded into polycarbonate filters (3×104 cells; 200 µL; Product #3464, Corning, NY, USA) coated with or without Matrigel (Product #354234, Corning, NY, USA). Then, 600 µL of DMEM containing 20% FBS was added to the lower chambers. After 22 h of incubation, the residual cells in the upper chamber were removed with cotton swabs. Cells on the chamber membrane were fixed with 4% paraformaldehyde for 20 min, and stained with 0.1% crystal violet for 15 min. We captured 5 random fields of each chamber using a light microscope.
Western blot
Total protein was extracted from cell lines or tissues with radioimmunoprecipitation assay (RIPA) lysis buffer containing phenylmethylsulfonyl fluoride (PMSF; RIPA: PMSF =100:1). The tissue lysates were centrifuged at 12,000 rpm for 10 min at 4 ℃, and the supernatants were separated for further analysis. Bicinchoninic (BCA) assay (Pierce, Thermo Fisher Scientific, USA) was applied to determine the protein concertation. Protein samples (20 μg protein/lane) were separated using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Billerica, MA, USA). The membrane was blocked in Tris-buffered saline containing 5% nonfat milk for 2 h at room temperature and then incubated overnight with primary antibodies at 4 ℃. After the membranes were washed 3 times with tris-buffered saline with Tween 20 (TBST), secondary antibodies were applied for 2 h at room temperature. After rinsing, the proteins were detected by enhanced chemiluminescence (SuperSignalTM West Pico PLUS, Thermo Fisher Scientific, USA). The protein levels were quantified by densitometry and normalized to the corresponding β-Actin level.
Statistical analysis
All the statistical analyses were performed with SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Non-parametric Mann-Whitney U test was used to analyze the differences of MAPT-AS1 expression between the tumor and control group. A receiver operating characteristic (ROC) curve was generated using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). The Kaplan–Meier method and log-rank test were performed to analyze the survival curves and differences. Chi-square tests were used to analyze the relationships between MAPT-AS1 expression and clinicopathological characteristics. Statistical significance was shown as P<0.05.
Results
The overexpression of MAPT-AS1 of BC tissues in patients
In our previous study, we identified the MAPT-AS1 upregulation with the highest significance in the survival-related lncRNAs in BC, based on TCGA database using RNA sequencing data (1). Here, we firstly detected the expression levels of MAPT-AS1 in BC tissues derived from patients by qRT-PCR. We detected a significantly higher level of MAPT-AS1 in BC tissues compared with normal group (Figure 1A). We also investigated the correlation between MAPT-AS1 expression and the clinicopathological parameters of BC patients. The result suggested that overexpression of MAPT-AS1 in BC tissues was significantly related to tumor size (P=0.005) and grade (P=0.023) (Table 1). Moreover, we further evaluated the expression levels of MAPT-AS1 in 5 different BC cell lines by qRT-PCR, including T47D, MCF-7, ZR-75-1, SKBR-3, and MDA-MB-231. Normal human mammary epithelial cell line MCF-10A was set as the control group. Similarly, overexpression MAPT-AS1 was detected in all 5 BC cell lines (Figure 1B). These results revealed that MAPT-AS1 is highly expressed in BC and has a close relationship with clinicopathological parameters such as tumor size and tumor grade.
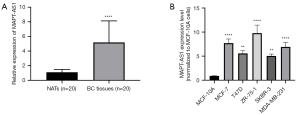
Table 1
| Clinicopathological features | n | MAPT-AS1 | χ2 | P | |
|---|---|---|---|---|---|
| Low expression | High expression | ||||
| Age, n (%) | 1.818 | 0.370 | |||
| ≤52 years | 9 | 6 (66.7) | 3 (33.3) | ||
| >52 years | 11 | 4 (36.4) | 7 (63.6) | ||
| Size, n (%) | 9.899 | 0.005** | |||
| ≤3 cm | 11 | 9 (81.8) | 2 (18.2) | ||
| >3 cm | 9 | 1 (11.1) | 8 (88.9) | ||
| Tumor grade, n (%) | 7.200 | 0.023* | |||
| I and II | 10 | 7 (70.0) | 3 (30.0) | ||
| III | 10 | 1 (10.0) | 9 (90.0) | ||
| Lymphatic metastasis, n (%) | 1.818 | 0.370 | |||
| Yes | 9 | 6 (66.7) | 3 (33.3) | ||
| No | 11 | 4 (36.4) | 7 (63.6) | ||
| Estrogen receptor, n (%) | 0.220 | 1.000 | |||
| Negative | 7 | 4 (57.1) | 3 (42.9) | ||
| Positive | 13 | 6 (46.2) | 7 (53.8) | ||
| Progesterone receptor, n (%) | 0.220 | 1.000 | |||
| Negative | 7 | 4 (57.1) | 3 (42.9) | ||
| Positive | 13 | 6 (46.2) | 7 (53.8) | ||
| HER2, n (%) | 0.800 | 0.656 | |||
| Negative | 10 | 6 (60.0) | 4 (40.0) | ||
| Positive | 10 | 4 (40.0) | 6 (60.0) | ||
| Ki-67, n (%) | 1.250 | 0.582 | |||
| ≤14 | 4 | 3 (75.0) | 1 (25.0) | ||
| >14 | 16 | 7 (43.8) | 9 (56.2) | ||
*, P <0.05; **, P<0.01. BC, breast cancer; HER2, human epidermal growth factor receptor 2.
The overexpression of serum MAPT-AS1 in BC patients
In order to analyze the serum MAPT-AS1 level in BC patients, we performed a series of quality control experiments prior to the qRT-PCR. Due to the good linearity and stability, 18S was determined as internal reference (Table S1). Then, qRT-PCR was applied to detect the serum MAPT-AS1. We firstly evaluated the reliability and stability of qRT-PCR for serum detection. The MAPT-AS1 cDNA was diluted 10 times, and the qRT-PCR results showed that the R2 of MAPT-AS1 standard curve was 0.988, R2 of internal reference 18S standard curve was 0.991, suggesting that qRT-PCR could detect different concentrations of serum lncRNAs (Figure S1A,S1B).
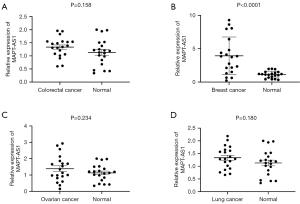
Based on the parameters screened above, qRT-PCR was used to detect the serum MAPT-AS1 from BC patients, lung cancer patients, ovarian cancer patients, colorectal cancer patients, and healthy people (n=20, respectively). As shown in Figure 2A-2D, MAPT-AS1 expression in serum of BC patients was significantly increased (P<0.0001), no significant differences of serum MAPT-AS1were found in patients with colorectal cancer patients, ovarian cancer patients, and lung cancer patients, indicating that MAPT-AS1 may be regarded as a new biomarker for BC patients.
The relationship between clinicopathological parameters and serum MAPT-AS1 in BC patients
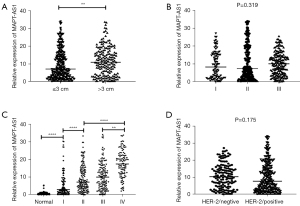
To investigate the relationship between the MAPT-AS1 expression level and clinicopathological parameters, the BC patients were classified into a high MAPT-AS1 group and a low MAPT-AS1 group according to the MAPT-AS1 expression level. As shown in Table 2, serum MAPT-AS1 expression was closely related to the tumor size, pathological grade, stage, and HER-2 expression in BC patients (P<0.05). Further analyses indicated that the serum MAPT-AS1 expression was significantly upregulated in the BC group with tumor tissue >3 cm than ≤3 cm (Figure 3A, P<0.01). No significant difference of serum MAPT-AS1 was found in different pathological grading groups (Figure 3B). In addition, the serum MAPT-AS1 was significantly upregulated in BC patients with stage I–IV comparing with the healthy group, and the expression was higher in patients with advanced BC than early-stage BC (Figure 3C, P<0.0001). No significant difference of serum MAPT-AS1 was found between the HER-2 negative and positive group (Figure 3D).
Table 2
| Clinicopathological parameters | n | MAPT-AS1 | χ2 | P | |
|---|---|---|---|---|---|
| Low expression | High expression | ||||
| Age, n (%) | 0.851 | 0.356 | |||
| ≤52 years | 300 | 150 (50.0) | 150 (50.0) | ||
| >52 years | 198 | 108 (54.5) | 90 (45.5) | ||
| Size, n (%) | 13.046 | <0.0001**** | |||
| ≤3 cm | 318 | 179 (56.3) | 139 (43.7) | ||
| >3 cm | 180 | 71 (39.4) | 109 (60.6) | ||
| Grade, n (%) | 5.169 | 0.023* | |||
| I and II | 302 | 164 (54.3) | 138 (45.7) | ||
| III | 196 | 86 (43.9) | 110 (56.1) | ||
| Stage, n (%) | |||||
| I/II | 252 | 172 (69.1) | 77 (30.9) | 70.973 | <0.0001**** |
| III/IV | 246 | 78 (31.3) | 171 (68.7) | ||
| Lymphatic metastasis, n (%) | 2.924 | 0.087 | |||
| Yes | 210 | 96 (45.7) | 114 (54.3) | ||
| No | 288 | 154 (53.5) | 134 (46.5) | ||
| Estrogen receptor, n (%) | 0.064 | 0.8 | |||
| Negative | 158 | 78 (49.4) | 80 (50.6) | ||
| Positive | 340 | 172 (50.6) | 168 (49.4) | ||
| Progesterone receptor, n (%) | 0.004 | 0.948 | |||
| Negative | 174 | 87 (50.0) | 87 (50.0) | ||
| Positive | 324 | 163 (50.3) | 161 (49.7) | ||
| HER2, n (%) | 6.368 | 0.012* | |||
| Negative | 145 | 60 (41.4) | 85 (58.6) | ||
| Positive | 353 | 190 (53.8) | 163 (46.2) | ||
| Ki-67, n (%) | 0.727 | 0.394 | |||
| ≤14 | 135 | 72 (53.3) | 63 (46.7) | ||
| >14 | 363 | 178 (49.0) | 185 (51.0) | ||
*, P <0.05; ****, P<0.0001. BC, breast cancer; HER2, human epidermal growth factor receptor 2.
The diagnostic efficiency of serum MAPT-AS1 in BC patients
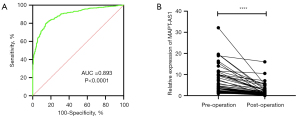
To study the diagnostic efficiency of MAPT-AS1, 498 BC patients and 429 normal people were analyzed. The diagnostic efficacy of MAPT-AS1 was estimated by ROC curve. The area under the curve (AUC) of serum MAPT-AS1 for BC detection was 0.893 (Figure 4A). The best cutoff value of serum MAPT-AS1 for predicting BC was 3.269 (fold change in BC compared with normal control groups, with a sensitivity of 86% and a specificity of 78.4%, suggesting that serum MAPT-AS1 was expected to be a serum biomarker for the diagnosis of BC). Besides, the correlation of serum MAPT-AS1 expression level in BC patients before and after surgery was explored. The results showed that the expression of MAPT-AS1 significantly decreased after the operation (Figure 4B), indicating that MAPT-AS1 may be applied as a cancer surveillance biomarker.
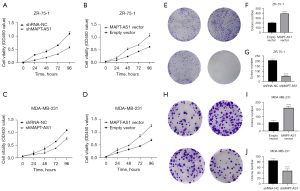
Effects of MAPT-AS1 on proliferation and colony formation of BC cells in vitro
Upregulated and downregulated MAPT-AS1 BC cell lines including ZR-75-1 and MDA-MB-231 were successfully established (Figure S2A-S2F). To investigate the potential effects of MAPT-AS1 on BC cell proliferation and mobility, CCK-8 assay and colony formation were applied. It was shown that cell proliferation was significantly increased in the MAPT-AS1 overexpression cells, and dramatically inhibited in the MAPT-AS1 knockdown cells by CCK-8 assay (Figure 5A-5D). Also, the colony formation capacity was elevated in the MAPT-AS1 overexpression cells but suppressed in the knockdown cells (Figure 5E-5J).
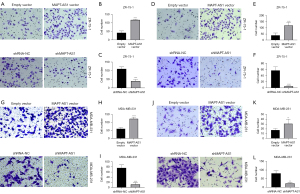
Effects of MAPT-AS1 on migration and invasion of BC cells in vitro
Transwell assay was used to investigate the effects of MAPT-AS1 on migration and invasion of BC cell lines including ZR-75-1 and MDA-MB-231. As shown in Figure 6A,6B, in the Transwell migration assay, the cell numbers in the lower compartment were significantly increased in MAPT-AS1 overexpression group, compared with empty vector group. Conversely, the cell numbers in the lower compartment were significantly reduced in MAPT-AS1 downregulation group. Similarly, the MAPT-AS1 overexpression cells promoted invasion compared to the control cells, while MAPT-AS1 knockdown cells showed the opposite results (Figure 6C-6L).
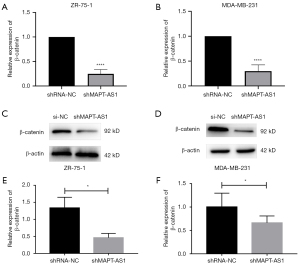
MAPT-AS1 overexpression inhibits the expression level of β-catenin in BC cell lines
To further explore the molecular mechanism of MAPT-AS1 in promoting the proliferation and migration of BC cells, we analyzed the effect of MAPT-AS1 on the Wnt/β-catenin signaling pathway. The qRT-PCR results showed a significantly reduced messenger RNA (mRNA) level of β-catenin in ZR-75-1 and MDA-MB-231 cell lines transfected with shMAPT-AS1 (Figure 7A,7B). In line with the mRNA results, the β-catenin protein level was also inhibited in BC cell lines after transfecting with shMAPT-AS1 (Figure 7C-7F).
Discussion
As one of the most common malignant tumors in women worldwide, BC has the clinical characteristics of concealed onset, common metastasis, and high degree of malignancy (14). The prognosis of BC patients is usually poor, seriously endangering the health of women, thus a new strategy for BC diagnosis and treatment is urgently needed (15). In the present study, we identified that MAPT-AS1 expression was significantly up-regulated in tissues and serum of BC patients. The overexpression of serum MAPT-AS1 was related to the larger tumor size, higher grade, tumor-node-metastasis (TNM) stages, and positive HER-2 expression. What’s more, ex vivo experiments showed that overexpression of MAPT-AS1 activates the Wnt/β-catenin signaling pathway, promoting proliferation, migration, and invasion of BC cell lines. Analysis of the ROC curve showed that MAPT-AS1 had a large AUC of 0.893, indicating MAPT-AS1 may be applied as a cancer surveillance biomarker with good diagnostic efficiency. The identification of MAPT-AS1 overexpression in BC patients could alert the physicians to make more positive and individualized treatment strategies in the clinic.
The lncRNAs play roles in chromatin remodeling, transcription, and post-transcription during the tumor progression (16). Recently, emerging study (17) has shown that lncRNAs regulate the characteristics of cancer cells, such as tumor proliferation, angiogenesis, invasion, and metastasis by activating or inhibiting specific signaling pathways in BC. It is identified that several lncRNAs positively regulate the cell proliferation of BC, including lncRNA SPRY4 intron transcript 1, DANCR, PVT1, CCAT1, and KCNQ1OT1; on the contrary, some lncRNAs play tumor-suppressor effects in BC cell proliferation, such as LINC01355, lncRNA SONE and lncRNA MAGI2-AS3 (18). The tissue and cell-type specificity makes lncRNAs attractive as diagnostic biomarkers, prognostic factors, and specific therapeutic targets.
The lncRNA MAPT-AS1 is an 840bp lncRNA transcribed from chromosome 17, existing in the antisense chain of MAPT promoter region (19). It is identified that MAPT-AS1 is involved in the occurrence and development of BC, and its expression varies in different BC types and stages. Wang et al. (11) demonstrated that MAPT-AS1 is overexpressed in BC but not in TNBC; however, high expression of MAPT-AS1 was correlated with better patient survival, suggesting MAPT-AS1 may play a role and be a potential survival predictive biomarker in BC. Another study analyzed the effect of MAPT-AS1 on proliferation and migration in ER-negative breast cancers, indicating that ER-negative patients with younger age (<60 cm), larger tumors (≥2 cm), metastatic lymph nodes, and stages (III–IV) had higher expression of MAPT-AS1 (12). In this study, we confirmed the overexpression of MAPT-AS1 in BC patient tissues and BC cell lines, which is in line with previous reports (11,12). Importantly, the serum MAPT-AS1 of BC patients was also detected. We found that MAPT-AS1 expression in serum of BC patients was significantly increased, and serum MAPT-AS1 expression was closely related to the tumor size, pathological grade, stage, and HER-2 expression in BC patients, indicating MAPT-AS1 may be considered as a potential biomarker for risk stratification and local regional metastasis in patients with BC.
Based on the above results, we further explored the regulative role of MAPT-AS1 in BC progression and potential molecular mechanism, the proliferation, migration, and invasion of BC cell lines ex vivo were evaluated. Cell proliferation was significantly increased in the MAPT-AS1 overexpression cells, and dramatically inhibited in the MAPT-AS1 knockdown cells. Besides, the MAPT-AS1 overexpression cells promoted migration and invasion compared to the control cells, while the MAPT-AS1 knockdown cells showed the opposite results. Therefore, MAPT-AS1 acts as an oncogenic molecule in BC progression and metastasis. Zhu et al. (20) identified a lncRNA signature to predict BC survival using bioinformatics and statistical methods, including PVT1, MAPT-AS1, LINC00667, and LINC00938, and they found this 4-lncRNA signature can be regarded as a potential prognostic biomarker for BC that may be relevant for clinical application. To date, the underlying mechanism of MAPT-AS1 involved in BC development remains elusive.
The Wnt/β-catenin signaling pathway takes part in several regulative processes of tumors, such as cell proliferation, migration, metabolism, and other physiological processes during embryonic development (21). The analyses of genome-wide sequencing and gene expression profiles have shown that the Wnt/β-catenin pathway has an essential role in BC proliferation and metastasis via immune microenvironment regulation, stemness maintenance, therapeutic resistance, phenotype shaping, and so on (22). Further, β-catenin can bind to the cytoplasmic tail of E-cadherin for cell-cell adhesion, mediating the migration and invasion processes (23). Here, we found significantly reduced mRNA level of β-catenin in ZR-75-1 and MDA-MB-231 cell lines transfected with shMAPT-AS1, validating the relationship between β-catenin and MAPT-AS1.
Altogether, we elucidated the abnormal upregulation of MAPT-AS1 in BC, which has roles in BC cell proliferation, migration, and invasion through activating the Wnt/β-catenin signaling pathway. The concentration of serum MAPT-AS1 was closely related to the large tumor size, grade, TNM stages, and HER-2 expression status of BC patients, thus can be considered as a reliable diagnostic marker for BC.
Acknowledgments
Funding: The study was supported by the Shunde Hospital of Guangzhou University of Chinese Medicine (No. 2019A1515011960).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-719/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-719/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-719/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committee of the Shunde Hospital of Guangzhou University of Chinese Medicine (No. KY-2020002) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coughlin SS. Epidemiology of Breast Cancer in Women. Adv Exp Med Biol 2019;1152:9-29. [Crossref] [PubMed]
- Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat 2016;159:395-406. [Crossref] [PubMed]
- Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134-50. [Crossref] [PubMed]
- Cai P, Otten ABC, Cheng B, et al. A genome-wide long noncoding RNA CRISPRi screen identifies PRANCR as a novel regulator of epidermal homeostasis. Genome Res 2020;30:22-34. [Crossref] [PubMed]
- Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol 2018;19:143-57. [Crossref] [PubMed]
- Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 2016;17:47-62. [Crossref] [PubMed]
- Zhang Q, Li T, Wang Z, et al. lncRNA NR2F1-AS1 promotes breast cancer angiogenesis through activating IGF-1/IGF-1R/ERK pathway. J Cell Mol Med 2020;24:8236-47. [Crossref] [PubMed]
- Crudele F, Bianchi N, Reali E, et al. The network of non-coding RNAs and their molecular targets in breast cancer. Mol Cancer 2020;19:61. [Crossref] [PubMed]
- Zhao W, Geng D, Li S, et al. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med 2018;7:842-55. [Crossref] [PubMed]
- Zhang M, Wang N, Song P, et al. LncRNA GATA3-AS1 facilitates tumour progression and immune escape in triple-negative breast cancer through destabilization of GATA3 but stabilization of PD-L1. Cell Prolif 2020;53:e12855. [Crossref] [PubMed]
- Wang D, Li J, Cai F, et al. Overexpression of MAPT-AS1 is associated with better patient survival in breast cancer. Biochem Cell Biol 2019;97:158-64. [Crossref] [PubMed]
- Pan Y, Pan Y, Cheng Y, et al. Knockdown of LncRNA MAPT-AS1 inhibites proliferation and migration and sensitizes cancer cells to paclitaxel by regulating MAPT expression in ER-negative breast cancers. Cell Biosci 2018;8:7. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Liang Y, Zhang H, Song X, et al. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol 2020;60:14-27. [Crossref] [PubMed]
- Waks AG, Winer EP. Breast Cancer Treatment. JAMA 2019;321:316. [Crossref] [PubMed]
- Huarte M. The emerging role of lncRNAs in cancer. Nat Med 2015;21:1253-61. [Crossref] [PubMed]
- Volovat SR, Volovat C, Hordila I, et al. MiRNA and LncRNA as Potential Biomarkers in Triple-Negative Breast Cancer: A Review. Front Oncol 2020;10:526850. [Crossref] [PubMed]
- Jin H, Du W, Huang W, et al. lncRNA and breast cancer: Progress from identifying mechanisms to challenges and opportunities of clinical treatment. Mol Ther Nucleic Acids 2021;25:613-637. [Crossref] [PubMed]
- Coupland KG, Kim WS, Halliday GM, et al. Role of the Long Non-Coding RNA MAPT-AS1 in Regulation of Microtubule Associated Protein Tau (MAPT) Expression in Parkinson's Disease. PLoS One 2016;11:e0157924. [Crossref] [PubMed]
- Zhu M, Lv Q, Huang H, et al. Identification of a four-long non-coding RNA signature in predicting breast cancer survival. Oncol Lett 2020;19:221-8. [PubMed]
- Bugter JM, Fenderico N, Maurice MM. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer 2021;21:5-21. [Crossref] [PubMed]
- Xu X, Zhang M, Xu F, et al. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer 2020;19:165. [Crossref] [PubMed]
- Benthani FA, Herrmann D, Tran PN, et al. 'MCC' protein interacts with E-cadherin and β-catenin strengthening cell-cell adhesion of HCT116 colon cancer cells. Oncogene 2018;37:663-72. [Crossref] [PubMed]
(English Language Editor: J. Jones)


