
Expanding landscape of CDKN1A (p21) functions: CDKN1A-mediated radioresistance of dermal Langerhans cells and its impact on the immune system
The CDKN1A (p21WAF1/CIP1) protein is the founding member of the CIP/KIP family of cyclin-dependent kinase (CDK) inhibitors. It is a p53 transcriptional target that plays a pivotal role in the DNA damage surveillance network through activating cell cycle checkpoints, promoting DNA repair, downregulating apoptosis, and triggering a senescence-like growth arrested response (premature senescence) (1-3). The anti-apoptotic property of CDKN1A is not only associated with its ability to halt cell-cycle progression and facilitate DNA repair, but also relies on its ability to inhibit the activity of proteins directly involved in the induction of apoptosis (e.g., the caspase cascade) and to control transcription, resulting in downregulation of pro-apoptotic genes and upregulation of genes with anti-apoptotic activities (1). In addition, we recently provided evidence suggesting that CDKN1A can positively regulate wild-type p53-induced phosphatase 1 (WIP1) (4), an anti-apoptotic phosphatase that inhibits p53 and its upstream kinases (e.g., ATM; CHK2). Consistent with these properties of CDKN1A, treatment of p53 wild-type solid tumor-derived cells with ionizing radiation or chemotherapeutic agents results in sustained upregulation of CDKN1A, protection against apoptotic cell death, and growth arrest through premature senescence (1).
In an elegant study recently published in Nature Immunology (5), Price and colleagues demonstrated a pivotal role for CDKN1A in inhibiting the apoptotic response of mouse epidermal Langerhans cells (LCs) following a total-body exposure to ionizing radiation; these LCs can subsequently migrate to the skin-draining lymph nodes and promote the expansion of regulatory T (Treg) cells (Figure 1). Zitvogel and Kroemer have published a News and Views article on the work done by this group in the same journal issue (6).
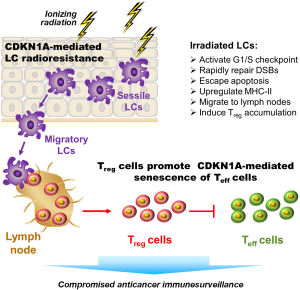
LCs are a subset of mononuclear phagocytes that form a dense network in the barrier surfaces, including the epidermis of the skin, and are long-lived, can divide, and replenish themselves. These cells tolerate relatively high doses of ionizing radiation and promote moderation of the immune surveillance system. Although sessile immature LCs reside in the epidermis, they are dynamic cells that can migrate to skin-draining lymph nodes where they influence the immune response. LCs require the chemokine receptor CCR7 for migrating to the lymph nodes (7).
Using a series of knockout and adoptive-transfer technologies, Price et al. revealed that the remarkable radioresistance phenotype of LCs is directly associated with high expression of CDKN1A, both endogenous and radiation-induced. Specifically, wild-type (CDKN1A-expressing) LCs exhibited resistance toward radiation-induced apoptosis as a consequence of CDKN1A-mediated activation of the G1/S cell cycle checkpoint coupled with rapid rejoining of DNA double-strand breaks (DSBs). Instead of undergoing apoptosis following irradiation, some wild-type LCs upregulated major histocompatibility complex (MHC) class II molecules, migrated to the skin-draining lymph nodes in a CCR7-dependent manner, and caused an increase in Treg cell numbers, which are known to suppress the immune response through targeting effector T cells. In contrast to wild-type LCs, CDKN1A-deficient (knockout) LCs underwent apoptosis post-irradiation and were thus unable to cause the accumulation of Treg cells in draining lymph nodes.
Price et al. (5) further demonstrated an important immunological consequence of these events. They found that the growth of subcutaneously injected malignant B16 melanoma or EL4 lymphoma cells was accelerated in irradiated (versus non-irradiated control) host mice bearing wild-type LCs, but this effect was not seen in mice bearing CDKN1A-deficient LCs or MHC class II–deficient LCs. The radiation-enhanced tumor growth was accompanied by increased numbers of Treg cells in the tumor and tumor-draining lymph nodes.
The impact of CDKN1A on the immune surveillance network is not limited to LCs. Ye et al. (8), for example, reported that one mechanism by which Treg cells suppress host immunity is by inducing CDKN1A-dependent senescence of responder naive and effector T cells. The mechanism by which CDKN1A provides a survival signal in one cell type (e.g., epidermal LCs) and a growth inhibitory (senescence) signal in another (e.g., naive/effector T cells) remains to be elucidated. However, these intriguing discoveries with hematopoietic cells, in concert with those reported previously with fibroblastic and epithelial cells (1), underscore the conclusion drawn by us (1,4) and by Warfel and El-Deiry (2) that a better understanding of the complexity of CDKN1A-mediated responses in different types of cells and tissues is crucial to determining whether modulating CDKN1A signaling might be a useful approach to the treatment of certain types of malignancies.
The findings reported by Price et al. (5) suggest an important role for CDKN1A in the expansion of Treg cells in response to total body irradiation that results in an immune-suppressed phenotype and a growth advantage for cancer cells. Such effects have also been widely exploited for the engraftment of non-self tissues into humans and also into animals, e.g., to generate mouse models of cancer. The question of whether LC-mediated immune suppression might impact negatively on the outcome of cancer radiotherapy was also raised by Price and colleagues. However, as these authors pointed out, radiotherapy to cancer patients is given very differently than the total-body exposures used in their study, notably with the dose being highly tailored to the tumor with maximal avoidance of normal tissue elements. We suspect that LC-mediated effects on the immune system will be much less important under such conditions, but this will require confirmation using small-animal image-guided radiotherapy platforms that better simulate the clinical situation (9).
Acknowledgments
Funding: The authors have continuously been supported by the Canadian Breast Cancer Foundation-Prairies/NWT region.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.12.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mirzayans R, Andrais B, Scott A, et al. Ionizing radiation-induced responses in human cells with differing TP53 status. Int J Mol Sci 2013;14:22409-35. [PubMed]
- Warfel NA, El-Deiry WS. p21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol 2013;25:52-8. [PubMed]
- Murray D, Mirzayans R. Role of therapy-induced cellular senescence in tumor cells and its modification in radiotherapy: the good, the bad and the ugly. J Nucl Med Radiat Ther 2013;S6:018.
- Mirzayans R, Andrais B, Scott A, et al. Spontaneous γH2AX foci in human solid tumor-derived cell lines in relation to p21WAF1 and WIP1 expression. Int J Mol Sci 2015;16:11609-28. [PubMed]
- Price JG, Idoyaga J, Salmon H, et al. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat Immunol 2015;16:1060-8. [PubMed]
- Zitvogel L, Kroemer G. Subversion of anticancer immunosurveillance by radiotherapy. Nat Immunol 2015;16:1005-7. [PubMed]
- Villablanca EJ, Mora JR. A two-step model for Langerhans cell migration to skin-draining LN. Eur J Immunol 2008;38:2975-80. [PubMed]
- Ye J, Huang X, Hsueh EC, et al. Human regulatory T cells induce T-lymphocyte senescence. Blood 2012;120:2021-31. [PubMed]
- Verhaegen F, Granton P, Tryggestad E. Small animal radiotherapy research platforms. Phys Med Biol 2011;56:R55-83. [PubMed]


