
A circadian prelude to regulation of angiogenesis and thrombosis by prolactin and plasminogen activator inhibitor-1
Angiogenesis is key for tissue growth and remodeling both during development, regeneration and under pathological situations such as in cancer (1). Angiogenesis is tightly regulated by a multitude of both pro-and anti-angiogenic factors, the balance of which—known as the angiogenic switch—is determining growth or quiescence of the vasculature (1). As such, endogenous angiogenesis inhibitors such as angiostatin, endostatin, thrombospondin and 16K prolactin are crucial, especially in adult organisms, for maintaining vascular quiescence and health (2). Characteristic for endogenous angiogenesis inhibitors is that they are produced by proteolytic cleavage of extra-cellular pro-angiogenic factors but the mechanisms regulating this process as well as the mechanisms by which they inhibit pro-angiogenic signaling remains poorly defined (2). In large part due to these difficulties, research on endogenous angiogenesis inhibitors have recently lost momentum in favor of efforts towards identifying ways of medically blocking pro-angiogenic factor signaling. However, the rather unsatisfying clinical results gained from inhibition of the main pro-angiogenic signaling pathway; the vascular endothelial growth factor (VEGF)—VEGF receptor axis in several types of cancer, have again raised attention to alternative ways of interfering with this critical aspect of tumor biology.
In this context, understanding the mechanisms of action of the endogenous anti-angiogenic factors are of pivotal importance. One such interesting anti-angiogenic factor is the 16 kDa, N-terminal fragment of prolactin (16K PRL or vasoinhibin). Prolactin is a pro-angiogenic protein that is efficiently processed to its 16K antiangiogenic fragment by bone-morphogenetic protein 1 (3) or cathepsin D (4), but whether other proteases also play a role in the processing, the mechanism behind its regulation as well as that leading to anti-angiogenic effects of 16K PRL, is poorly understood. Recently, in an article published in Nature Medicine (5), Struman and collegues discovered a key piece of the puzzle: Surprisingly, the anti-angiogenic effects of 16K PRL was found to depend on interaction with the otherwise pro-angiogenic plasminogen activator inhibitor type 1 (PAI-1) in complex with urokinase-type plasminogen activator (uPA) and the uPA receptor (uPAR). The authors found that 16K PRL lost all anti-angiogenic functions in mice lacking PAI-1 or where uPAR signaling was inhibited (5). This finding is particularly interesting as PAI-1, uPA and uPAR are considered pro-angiogenic factors (6), indicating that 16K PRL may not only disable such pro-angiogenic signaling but at the same time even render anti-angiogenic signals through this pathway indicating the discovery of a potential double-edged sword that could disrupt tumor angiogenesis by dual mechanisms the same time. As this entire regulation may be achieved outside of the cell (Figure 1), pharmacological targeting of this pathway even by large molecules such as antibodies or recombinant proteins (for example 16K PRL itself or proteases that may cleave PRL to 16K PRL) could be feasible. This report may therefore constitute the core of a new way of targeting tumor angiogenesis, but many pertinent questions remains to be answered before such an impact can be practically achieved. For example, which are the proteases involved in the processing from pro-angiogenic full- length PRL to the anti-angiogenic 16K PRL, and how is the proteolysis of PRL regulated? How is the active 16K PRL-PAI-uPA-uPAR-LRP complex constructed? Which of these constituents are driving the anti-angiogenic effects down-stream of 16K PRL and how? And, given that 16K PRL inhibits PAI (see below), and through this mechanism activate fibrinolytic functions of uPA, how is then the anti-angiogenic induction achieved and uncoupled from the pro-fibrinolytic activity of this pathway, considering that the anti-angiogenic effects relied on inhibition of uPA-uPAR signaling?
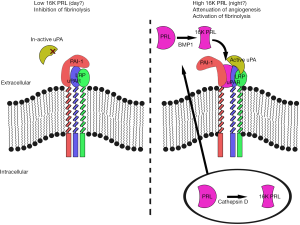
PAI-uPA-uPAR is not only involved in angiogenesis. Indeed the best characterized role of this pathway is in the regulation of fibrinolysis, a critical aspect of fibrin clot formation and resolution (7). Reduced activity of uPA leads to impaired fibrinolysis which may increase the risk for serious cardiovascular events including atherosclerosis and thromboembolisms (8). Importantly, Bajou et al. found that 16K PRL significantly reduced the inhibitory effects of PAI-1 leading to improved clearance of thrombi in experimental thrombus and thromboembolism models (5). This role of 16K PRL potentially extends beyond the importance it may have as an anti-angiogenic molecule and suggests that it may play important roles as a fibrinolytic agent and therefore potentially as an inhibitor of thrombus-related cardiovascular events such as myocardial infarction, pulmonary thromboembolism or stroke. It is well known that such cardiovascular events occur predominantly in the morning (9). Understanding the factors determining this difference has been a subject of intense research and a number of circadian factors playing important roles in this diurnal regulation have been identified (10). One of these is endothelial bone and muscle arnt-like 1 (Bmal1). Bmal1 is an important driver of the cellular circadian clock leading to the production of a wide range of factors regulating circadian functions of our organism (11). These include regulation of immune function, metabolism and alertness during the active period (day for humans, night for mice) (11). Bmal1 is also critically involved in regulation of the cardiovascular system including increased heart-rate and blood pressure during the active period (9). In addition to Bmal1, the negative translational feedback inhibitor Period2 (Per2), is important for maintaining rhythmicity in the circadian transcription-translation feedback loop, and also plays a major role in regulation of cardiovascular physiology and pathology (9-12). Interestingly, both Bmal1 and Per2 regulate the transcriptional frequency of the pai1 and prl genes (13,14), meaning that both PAI-1 and PRL are considered circadian genes. Indeed, PRL plays major roles in circadian endocrine regulation and as such in the communication between the light-sensitive circadian rhythms in the retino-hypothalamic-pituitary axis and peripheral tissues. PRL is intimately coupled to circadian rhythms of sex hormone production and function, reproductive physiology, metabolism (especially lipid metabolism) and regulation of the immune system (especially via corticosteroids) (15). Interestingly, PRL levels decrease in individuals engaged in frequent shift-work (16), a known risk factor for breast—and other cancers, indicating that night-time production of PRL and perhaps especially the 16K PRL isoform is important for reducing breast cancer risk. Less is known regarding circadian functions of PAI-1. However, PAI-1 is known to be produced at high levels in the morning and dramatically reduced in the evening (13). As PRL is produced both as the full length protein, which is inert in terms of regulating fibrinolysis, and as the 16K PRL fragment, found by Bajou et al. to render PAI-1 fibrinolytic rather than anti-fibrinolytic, this may suggest that nocturnal 16K PRL may be important for mitigating the anti-fibrinolytic effects of the rising levels of PAI-1 during the night, but that as the 16K PRL levels concurrently decrease in the early hours of the morning, thus leading to a tipping of the balance in favor of anti-fibrinolysis and increased risk of cardiovascular events—especially those dependent on thrombus formation—in the morning.
Recently it was discovered that also the prototypical angiogenic factor VEGF is under direct transcriptional regulation by Bmal1 and Per2 (17,18). Mutating the Bmal1-binding sites in the VEGF-promoter, but leaving other transcription factor binding sites untouched completely abolished VEGF-production during zebrafish embryogenesis and inhibited developmental angiogenesis (17). VEGF transcription was highest during the inactive phase, a finding that has been confirmed by others in humans (19). 16K PRL may therefore act as an important endogenous inhibitor of angiogenesis during the night, when VEGF- levels are at the highest level, thus mediating a controlled vascular growth response and avoiding exaggerated neovascularization and vascular permeability (Figure 1). Indeed, evidence for a strong role of the circadian clock in regulation of angiogenesis, including tumor angiogenesis, is growing. The tumor angiogenic process itself, as well as the effectivity resulting from anti-angiogenic therapy differs dramatically during the day and during the night coupled to the changes in circulating levels of angiogenic factors and the molecular control of angiogenic factors such as eNOS in endothelial cells themselves by Bmal1 and Per2 (11,12,18). On this background, and considering the well-established circadian regulation and functions of PRL, it is tempting to speculate that disruption of night-time 16K PRL production may occur and drive increased cancer (especially breast cancer) incidence in night-shift workers. If so, could treatment with 16K PRL in the evening, be an attractive avenue for anti-angiogenic treatment in shift-workers? These interesting potential implications of the work by Bajou et al. deserved further investigation.
Acknowledgments
Funding: Dr. Jensen’s lab is supported by the Swedish Society for Medical Research (SSMF), The Ollie and Elof Ericsson’s Foundation and the Swedish Research Council (VR).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2016.01.06). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jensen LD, Rouhi P, Cao Z, et al. Zebrafish models to study hypoxia-induced pathological angiogenesis in malignant and non-malignant diseases. Birth Defects Res C Embryo Today 2011;93:182-93. [PubMed]
- Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk Res 2009;33:638-44. [PubMed]
- Ge G, Fernández CA, Moses MA, et al. Bone morphogenetic protein 1 processes prolactin to a 17-kDa antiangiogenic factor. Proc Natl Acad Sci U S A 2007;104:10010-5. [PubMed]
- Khurana S, Liby K, Buckley AR, et al. Proteolysis of human prolactin: resistance to cathepsin D and formation of a nonangiostatic, C-terminal 16K fragment by thrombin. Endocrinology 1999;140:4127-32. [PubMed]
- Bajou K, Herkenne S, Thijssen VL, et al. PAI-1 mediates the antiangiogenic and profibrinolytic effects of 16K prolactin. Nat Med 2014;20:741-7. [PubMed]
- Ulisse S, Baldini E. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets 2009;9:32-71. [PubMed]
- Myöhänen H, Vaheri A. Regulation and interactions in the activation of cell-associated plasminogen. Cell Mol Life Sci 2004;61:2840-58. [PubMed]
- Mackman N. Triggers, targets and treatments for thrombosis. Nature 2008;451:914-8. [PubMed]
- Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res 2010;106:833-41. [PubMed]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002;418:935-41. [PubMed]
- Jensen LD, Gyllenhaal C, Block K, et al. Circadian angiogenesis. Biomol Concepts 2014;5:245-56. [PubMed]
- Jensen LD. The circadian clock and hypoxia in tumor cell de-differentiation and metastasis. Biochim Biophys Acta 2015;1850:1633-41.
- Oishi K, Miyazaki K, Uchida D, et al. PERIOD2 is a circadian negative regulator of PAI-1 gene expression in mice. J Mol Cell Cardiol 2009;46:545-52. [PubMed]
- Bose S, Boockfor FR. Episodes of prolactin gene expression in GH3 cells are dependent on selective promoter binding of multiple circadian elements. Endocrinology 2010;151:2287-96. [PubMed]
- Freeman ME, Kanyicska B, Lerant A, et al. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000;80:1523-631. [PubMed]
- Herichova I. Changes of physiological functions induced by shift work. Endocr Regul 2013;47:159-70. [PubMed]
- Jensen LD, Cao Z, Nakamura M, et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep 2012;2:231-41. [PubMed]
- Jensen LD, Cao Y. Clock controls angiogenesis. Cell Cycle 2013;12:405-8. [PubMed]
- Endo I, Mitsui T, Nishino M, et al. Diurnal fluctuation of edema synchronized with plasma VEGF concentration in a patient with POEMS syndrome. Intern Med 2002;41:1196-8. [PubMed]


