
Psychological resilience is related to postoperative adverse events and quality of life in patients with glioma: a retrospective cohort study
Introduction
Glioma is one of the most common cerebral malignancies worldwide (1), and can be divided into low- and high-grade gliomas based on the pathological characteristics, growth speed, and invasive ability of the tumors (2). The survival time of glioma patients varies depending on the grade of tumor. It has been reported that the survival time of patients with high-grade gliomas ranges from 3 to 10 years (3,4), while patients with low-grade gliomas have a better prognosis, with a survival time ranging from 7 to 15 years (5-7). At present, radical surgical resection combined with adjuvant radiotherapy or chemotherapy is considered to be the best treatment for glioma. However, the incidence of postoperative adverse events is as high as 18% to 71% (8), which significantly affects the postoperative recovery, prolongs the hospital stay, worsens the quality of life (QoL), and even increases the mortality of patients (9,10). Some risk factors were identified to increase the incidence of postoperative adverse events and worsen the QoL in glioma patients, such as grade of glioma, duration of surgical procedures and the existence of chronic diseases (5,8,9). However, very few studies were performed to determine the effects of emotional status on the postoperative adverse events and QoL in glioma patients.
Regardless of the grade of glioma, a certain proportion of patients will experience some adverse emotional reactions after being diagnosed and informed of their condition, which is known as reactive emotional disorder, and mainly manifests as depression and anxiety (11). There are numerous studies on the effects of reactive mood disorder on the survival time of glioma patients, but the results obtained differ and have not been unified (12-14). In recent years, studies have gradually recognized the important role of psychological resilience in patients with chronic diseases and malignant tumors (15-17). Psychological resilience refers to the ability of an individual to recover from negative experiences and flexibly adapt to a changing external environment. Psychological resilience is an effective index in evaluating the psychological status of patients (18). Previous studies have shown that better psychological resilience is positively correlated with better prognosis of patients, including in gastric, breast, and prostate cancers (19-21). However, at present, there are no studies analyzing the impact of psychological resilience on postoperative adverse events and QoL in patients with resectable gliomas.
Therefore, we conducted this retrospective cohort study in order to investigate the effect of preoperative psychological resilience on the incidence of postoperative adverse events and QoL of glioma patients. We collected the clinical data of patients with resectable gliomas over the last 4 years, and analyzed the effect of changes in preoperative psychological resilience on the postoperative outcome of these patients, so as to provide a basis for preoperative treatment and nursing. Our hypothesis is that good or improved preoperative psychological resilience is conducive to a reduction in the incidence of postoperative adverse events and an improvement in the QoL of glioma patients. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-732/rc).
Methods
Patient selection
This study was a retrospective cohort study and included glioma patients between March 2016 and July 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Affiliated Hospital of Nantong University (No. 2020-063). Individual consent for this retrospective analysis was waived. Patients were included according to the following criteria: (I) adult patients (18–80 years old), (II) patients receiving surgical resection, (III) patients with a postoperative pathological diagnosis of glioma. The following patients were excluded: (I) patients with insufficient perioperative data or those lost follow-up, (II) patients with other malignant tumors, (III) patients unable to read or answer the questionnaires below, (IV) patients participating in other clinical trials, which may affect the results of our study.
Data collection
After enrollment, the following demographic and clinical data of the included glioma patients were retrospectively reviewed by two investigators: gender, age, body mass index (BMI), spouse, educational level, employment status, duration of disease diagnosis, concomitant diseases (hypertension, smoking, diabetes, chronic lung disease, and chronic liver dysfunction), tumor position (frontal lobe, temporal lobe, parietal lobe, occipital lobe, multiple lobes, or other lobes), tumor location (left, right, or both), extent of resection (gross total resection or partial resection), World Health Organization (WHO) grade, histopathology (glioblastoma, low-grade glioma, or high-grade glioma), and Karnofsky performance scale.
Psychological resilience assessment
This study used the Connor-Davidson resilience scale (CD-RISC) to evaluate the psychological resilience of the included patients. The CD-RISC includes 25 questions, with a maximum of four points and a minimum of zero points for each question, and a total score of 100 points. These questions encompassed the three dimensions of tenacity, self-improvement, and optimism, with higher total scores representing better psychological resilience. In this study, CD-RISC scores >70 points were considered to indicate high psychological resilience. The psychological resilience of all included patients was assessed 1 day before surgery. For some patients who required hospitalization for more than 1 week before surgery, psychological resilience was also evaluated at day 1 after admission. By comparing the two scores, we divided the patients into an increased resilience group and a decreased resilience group, and performed subgroup analysis to determine the impact of psychological resilience changes on the prognosis of glioma patients during preoperative hospitalization.
Postoperative adverse events
All included patients were followed up for at least 6 months and the following postoperative adverse events within 1 month after surgery were recorded: neurological complications (such as motor deficit, speech impairment, visual impairment, and seizure), intracranial hemorrhage, cerebrovascular accident, postoperative ventilator use, acute kidney injury, and septic shock.
QoL assessment
All included patients were followed up within 3–6 months after surgery via telephone or Internet, and two questionnaires were completed to assess their postoperative QoL, including the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30 and the EORTC QLQ-BN20. The EORTC QLQ-C30 comprises five functional dimensions: physical functioning, role functioning, cognitive deficits, emotional functioning, and social functioning. It also consists of eight symptoms: fatigue, pain, nausea/vomiting, dyspnea, appetite loss, insomnia, constipation, and diarrhea. The EORTC QLQ-BN20 is designed for brain cancer and consists of four dimensions, including future uncertainty, visual disorder, motor dysfunction, and communication deficit. It also includes eight symptoms: seizures, headache, drowsiness, itchy skin, hair loss, weakness in legs, and bladder control issues. Patients with missing follow-up were excluded from this study.
Statistical analysis
In this study, all statistical analyses were performed using SPSS 20.0, and all figures were generated using GraphPad 7.0. Continuous variables were expressed as the mean and standard deviation, and the Student t-test was used for comparison between the two groups. Categorical variables were expressed as a number and percentage, and the chi-square test was used for comparison between the two groups. The associations between psychological resilience and postoperative adverse events/QoL were determined using multivariable logistic and linear regression analysis after adjusting other factors identified in univariable regression, respectively. A two-sided P value less than 0.05 was considered to indicate a statistically significantly difference.
Results
Figure 1 displays the inclusion of glioma patients in this study. A total of 381 out of 452 glioma patients were included, and their psychological resilience was assessed for grouping. There were 97 patients in the high resilience group and 284 patients in the low resilience group. The patients’ demographic and clinical data are shown in Table 1. We observed that there were significantly more male and younger patients in the high resilience group than in the low resilience group. In addition, considerably more patients in the high resilience group had a higher educational level (19.6% vs. 7.7%, P=0.001). No significant differences were observed in the other variables.
Table 1
| Variables | Total | High resilience group | Low resilience group | P value |
|---|---|---|---|---|
| Number | 381 | 97 | 284 | |
| Gender (male) | 188 (49.3%) | 57 (58.8%) | 131 (46.1%) | 0.032 |
| Age, mean ± SD | 61.4±13.8 | 58.9±12.1 | 62.2±14.3 | 0.037 |
| BMI, mean ± SD | 23.0±5.3 | 22.8±4.8 | 23.1±5.4 | 0.620 |
| Spouse | 339 (90.0%) | 87 (89.7%) | 252 (88.7%) | 0.795 |
| Educational level (college or above) | 41 (10.8%) | 19 (19.6%) | 22 (7.7%) | 0.001 |
| Employment status (employed) | 153 (40.2%) | 34 (35.1%) | 119 (41.9%) | 0.235 |
| Duration of disease, mean ± SD (month) | 25.3±7.5 | 25.6±7.7 | 25.2±6.7 | 0.689 |
| Concomitant diseases | ||||
| Hypertension | 100 (26.2%) | 20 (50.5%) | 80 (28.2%) | 0.144 |
| Smoking | 51 (13.4%) | 10 (10.3%) | 41 (14.4%) | 0.303 |
| Diabetes | 30 (7.9%) | 6 (6.2%) | 24 (8.5%) | 0.475 |
| Chronic lung disease | 10 (2.6%) | 3 (3.1%) | 7 (2.5%) | 0.738 |
| Chronic liver dysfunction | 28 (7.3%) | 6 (6.2%) | 22 (7.7%) | 0.611 |
| Tumor position | 0.201 | |||
| Frontal lobe | 126 (33.1%) | 26 (26.8%) | 100 (35.2%) | |
| Temporal lobe | 84 (22.0%) | 24 (24.7%) | 60 (21.1%) | |
| Parietal lobe | 69 (18.1%) | 15 (15.5%) | 54 (19.0%) | |
| Multiple lobes | 70 (18.4%) | 23 (23.7%) | 47 (16.5%) | |
| Other lobes | 32 (8.4%) | 9 (9.3%) | 23 (8.1%) | |
| Tumor location | 0.932 | |||
| Left | 183 (48.0%) | 48 (49.5%) | 135 (47.5%) | |
| Right | 189 (49.6%) | 47 (48.5%) | 142 (50.0%) | |
| Both | 9 (2.4%) | 2 (2.1%) | 7 (2.5%) | |
| Extent of resection | 0.141 | |||
| Gross total resection | 285 (74.8%) | 78 (80.4%) | 207 (72.9%) | |
| Partial resection | 96 (25.2%) | 19 (19.6%) | 77 (27.1%) | |
| WHO grade | 0.413 | |||
| II | 70 (18.4%) | 14 (14.4%) | 56 (19.7%) | |
| III | 189 (49.6%) | 48 (49.5%) | 141 (49.6%) | |
| IV | 122 (32.0%) | 35 (36.1%) | 87 (30.6%) | |
| Histopathology | 0.498 | |||
| Glioblastoma | 80 (21.0%) | 21 (21.6%) | 59 (20.8%) | |
| Low-grade glioma | 211 (55.4%) | 36 (37.1%) | 124 (43.7%) | |
| High-grade glioma | 90 (23.6%) | 40 (41.2%) | 101 (35.6%) | |
| Karnofsky performance scale, mean ± SD | 79.8±11.0 | 80.7±9.9 | 79.4±13.2 | 0.416 |
BMI, body mass index; WHO, World Health Organization.
Postoperative adverse events were recorded as shown in Table 2. The most common adverse events in this study were neurological complications (16.0%), followed by acute kidney injuries and cerebrovascular accidents. We also found that more neurological complications occurred in the low resilience group (18.7% vs. 8.2%, P=0.016). Also, more acute kidney injury occurred in the low resilience group, however, the difference was not statistically significant. The risk factors of neurological complications in glioma patients receiving surgical treatment were analyzed using multivariable logistic regression (as shown in Table 3). We identified advanced age and longer duration of disease as important risk factors of neurological complications in included patients, and patients with higher Karnofsky performance scale scores and psychological resilience were less prone to adverse events.
Table 2
| Variables | Total | High resilience group | Low resilience group | P value |
|---|---|---|---|---|
| Number | 381 | 97 | 284 | |
| Neurological complications | 61 (16.0%) | 8 (8.2%) | 53 (18.7%) | 0.016 |
| Intracranial hemorrhage | 9 (2.4%) | 3 (3.1%) | 6 (2.1%) | 0.412 |
| Cerebrovascular accident | 22 (5.8%) | 5 (5.2%) | 17 (6.0%) | 0.762 |
| Postoperative ventilator use | 17 (4.5%) | 5 (5.2%) | 12 (4.2%) | 0.702 |
| Acute kidney injury | 50 (13.1%) | 9 (9.3%) | 50 (17.6%) | 0.050 |
| Septic shock | 11 (2.9%) | 3 (3.1%) | 8 (2.8%) | 0.889 |
Table 3
| Variables | Multivariable logistic regression | |
|---|---|---|
| OR (95% CI) | P value | |
| Age | 1.042 (1.017 to 1.069) | 0.001 |
| Duration of disease | 1.050 (1.003 to 1.100) | 0.038 |
| Karnofsky performance scale | 0.956 (0.930 to 0.983) | 0.001 |
| High resilience vs. low resilience | 0.413 (0.184 to 0.927) | 0.032 |
OR, odds ratio; CI, confidence interval.
The QoL of included patients was assessed within 3–6 months using EORTC QLQ-C30 and EORTC QLQ-BN20, as shown in Figure 2. We observed that patients in the high resilience group had higher scores in global health status, physical functioning, role functioning, and cognitive functioning according to EORTC QLQ-C30. These patients also suffered less from nausea/vomiting and constipation. Patients in the low resilience group had more future uncertainty, motor dysfunction, headache, drowsiness, weakness in the legs, and bladder control issues according to EORTC QLQ-BN20. The risk factors of postoperative QoL (global health status) were assessed using multivariable linear regression, as shown in Table 4. We found that patients with advanced age and higher grade of tumor were prone to worse postoperative QoL, and higher educational level, Karnofsky performance scale scores, and psychological resilience acted as protective factors for postoperative QoL.

Table 4
| Variables | Multivariable linear regression | |
|---|---|---|
| β (95% CI) | P value | |
| Age | –0.172 (–0.282 to –0.062) | 0.002 |
| Karnofsky performance scale | 0.229 (0.113 to 0.344) | <0.001 |
| Higher educational level | 4.960 (0.800 to 9.119) | 0.020 |
| Higher tumor grade | –2.243 (–4.097 to –0.390) | 0.018 |
| High resilience vs. low resilience | 5.102 (2.152 to 8.052) | 0.001 |
QoL, quality of life; CI, confidence interval.
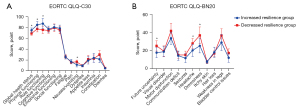
We performed a further subgroup analysis, and excluded patients who had only one assessment and two unchanged scores, based on whether the resilience scores of the patients increased or decreased during hospitalization. No significant differences were observed between the demographic and clinical data of the increased resilience group (n=26 patients) and the decreased resilience group (n=35 patients), as shown in Table 5. Also, the incidence of postoperative adverse events in these two groups are displayed in Table 6, and it was found that the incidence of neurological complications was markedly higher in the decreased resilience group compared to the increased resilience group (22.9% vs. 3.8%, P=0.039). No significant differences were found in the other adverse events. Finally, the EORTC QLQ-C30 and EORTC QLQ-BN20 scores in these two groups are shown in Figure 3. We observed that patients with increased resilience had better global health status, physical functioning, and role functioning, as well as less severe nausea/vomiting. Moreover, patients with increased resilience also had more future uncertainty, headache, and drowsiness.
Table 5
| Variables | Increased resilience group | Decreased resilience group | P value |
|---|---|---|---|
| Number | 26 | 35 | |
| Gender (male) | 14 (53.8%) | 15 (42.9%) | 0.395 |
| Age, mean ± SD | 60.7±12.1 | 61.9±12.1 | 0.479 |
| BMI, mean ± SD | 24.4±5.3 | 22.9±3.9 | 0.227 |
| Spouse | 23 (53.8%) | 31 (88.6%) | 0.989 |
| Educational level (college or above) | 4 (15.4%) | 4 (11.4%) | 0.651 |
| Employment status (employed) | 8 (30.8%) | 13 (37.1%) | 0.604 |
| Duration of disease , mean ± SD (month) | 24.6±8.1 | 24.2±5.8 | 0.803 |
| Concomitant diseases | |||
| Hypertension | 4 (15.4%) | 8 (22.9%) | 0.468 |
| Smoking | 2 (7.7%) | 4 (11.4%) | 0.628 |
| Diabetes | 1 (3.8%) | 1 (2.9%) | 0.830 |
| Chronic lung disease | 0 (0%) | 0 (0%) | – |
| Chronic liver dysfunction | 0 (0%) | 2 (5.7%) | 0.215 |
| Tumor position | 0.468 | ||
| Frontal lobe | 7 (26.9%) | 14 (40.0%) | |
| Temporal lobe | 7 (26.9%) | 4 (11.4%) | |
| Parietal lobe | 3 (11.5%) | 6 (17.1%) | |
| Multiple lobes | 7 (26.9%) | 7 (20.0%) | |
| Other lobes | 2 (7.7%) | 4 (11.4%) | |
| Tumor location | 0.575 | ||
| Left | 15 (57.7%) | 17 (48.6%) | |
| Right | 11 (42.3%) | 17 (48.6%) | |
| Both | 0 (0%) | 1 (2.9%) | |
| Extent of resection | 0.628 | ||
| Gross total resection | 23 (88.5%) | 30 (85.7%) | |
| Partial resection | 3 (11.5%) | 5 (14.3%) | |
| WHO grade | 0.725 | ||
| II | 2 (7.7%) | 5 (14.3%) | |
| III | 15 (57.7%) | 19 (54.3%) | |
| IV | 9 (34.6%) | 11 (31.4%) | |
| Histopathology | 0.711 | ||
| Glioblastoma | 3 (11.5%) | 4 (11.4%) | |
| Low-grade glioma | 13 (50.0%) | 14 (40.0%) | |
| High-grade glioma | 10 (38.5%) | 17 (48.6%) | |
| Karnofsky performance scale , mean ± SD | 80.9±12.8 | 80.0±13.8 | 0.582 |
BMI, body mass index; WHO, World Health Organization.
Table 6
| Variables | Increased resilience group | Decreased resilience group | P value |
|---|---|---|---|
| Number | 26 | 35 | |
| Neurological complications | 1 (3.8%) | 8 (22.9%) | 0.039 |
| Intracranial hemorrhage | 0 (0%) | 1 (2.9%) | 0.385 |
| Cerebrovascular accident | 1 (3.8%) | 4 (11.4%) | 0.286 |
| Postoperative ventilator use | 0 (0%) | 2 (5.7%) | 0.215 |
| Acute kidney injury | 3 (11.5%) | 7 (20.0%) | 0.301 |
| Septic shock | 0 (0%) | 2 (5.7%) | 0.215 |
Discussion
Psychological resilience plays an important role in the occurrence and development of various diseases, but its impact on glioma patients has not yet been elucidated. This is the first study to explore the effect of psychological resilience on the postoperative prognosis of glioma patients. Our results showed that good psychological resilience was helpful in reducing the incidence of neurological complications and improving the overall QoL at 3–6 months after glioma surgery. In addition, some patients' psychological resilience changes during hospitalization, and our study found that patients with increased psychological resilience scores during hospitalization had significantly better prognoses than those with decreased psychological resilience scores, which may provide some basis for the clinical nursing of glioma patients.
Although the preoperative evaluation and surgical procedures of gliomas have been greatly improved in recent years, the incidence of postoperative adverse events in glioma patients remains high (22). One study, which reviewed a number of other studies, pointed out that glioma patients often have some neurological dysfunction in the early postoperative stage, which gradually returns to normal or close to normal levels in the later stage (23). In our study, neurological complications were the most common postoperative adverse event within 1 month postoperatively, with an incidence of 16%. Interestingly, the incidence of postoperative neurological complications in patients with good preoperative psychological resilience was only 8.2%, while that in patients with poor psychological resilience was as high as 18.7%. According to multivariate logistic regression analysis, preoperative psychological resilience is an important risk factor for postoperative neurological complications. In addition, postoperative acute renal injury was also a common adverse event in our study, and its incidence in patients with low psychological resilience was higher, although the difference was not statistically significant (P=0.05). Most previous studies have reported that postoperative hydrocephalus or severe intracerebral hemorrhage was the most common cause of poor prognosis and even death in glioma patients (5,24). In this study, these adverse events occurred less, and thus, the role of psychological elasticity with such conditions was not identified.
Numerous studies have been carried out on the current situation of postoperative QoL in glioma patients (25-27). Teng et al. performed the largest prospective longitudinal study of the QoL of postoperative patients with low-grade glioma, and reported reduced global QoL at every 12-month interval (25). Other studies showed that glioma-related symptoms, age, and tumor grade could significantly affect the QoL of patients (26,27). Since our study was a retrospective study and considering the defects in the recording of glioma-related symptoms in patients, the symptoms were not included in this study. The severity of patients' symptoms was mainly indirectly reflected by the Karnofsky performance scale score.
The results also showed that age, Karnofsky performance scale score, education level, tumor grade, and psychological resilience were related to postoperative QOL. It has been reported that the main reason for the decline of QoL in elderly patients with glioma compared with young patients is that elderly patients have a more obvious decline in their physiological function (28). Educational level was also previously found to be associated with postoperative QoL in glioma patients (29). Kim et al. point out that interventions aimed at improving the QoL of glioma patients must consider the improvement of postoperative psychological resilience (30).
At present, there are no relevant studies on the effect of the change in psychological resilience on the QoL in glioma patients. One previous study reported that the changes of psychological resilience in patients with physical disabilities were significantly related to fatigue, sleep quality, and physiological function (31). Furthermore, the improvement of psychological elasticity has also been shown to be positively correlated with QoL in a variety of tumor patients (32). In our study, psychological resilience in some patients increased due to the psychological guidance of nurses and the enlightenment of family members during hospitalization, and psychological resilience in other patients decreased due to their worry about their own condition or familial factors. By comparing the prognosis of patients in these two subgroups, we observed that the incidence of postoperative neurological complications decreased and QoL was significantly improved significantly in patients with improved psychological resilience scores. This suggests the importance of psychological guidance and psychological dredging for glioma patients during hospitalization, and provides a basis for clinical nursing.
This study has some limitations that should be noted. Firstly, this study is a retrospective study, and thus, some variables cannot be completely collected, especially the symptoms during the patient's illness, which are related to the occurrence of postoperative adverse events and QoL. We used the Karnofsky performance scale score to indirectly reflect the severity of patients' symptoms, but there are still limitations in reflecting the patient's condition. Besides, baseline QoL of enrolled patients were not obtained and it may induce some bias in the results. Secondly, this study used the CD-RISC to evaluate the psychological resilience of patients. This scale is commonly used for the evaluation of psychological resilience, but is prone to obvious deviation due to patients’ subjective emotions and cannot objectively reflect their psychological resilience. Thirdly, since most patients were not hospitalized for an extended period preoperatively, and there are few patients who completed two resilience assessments, our subgroup analysis cannot provide a more in-depth analysis.
In conclusion, this retrospective study showed that preoperative psychological resilience plays an important role in the incidence of postoperative adverse events and QoL of glioma patients. Therefore, improving psychological resilience and preventing deterioration of psychological resilience in glioma patients prior to surgery should be a key measure of preoperative nursing.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-732/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-732/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-732/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Affiliated Hospital of Nantong University (No. 2020-063). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Kessel E, Huenges Wajer IMC, Ruis C, et al. Cognitive impairments are independently associated with shorter survival in diffuse glioma patients. J Neurol 2021;268:1434-42. [Crossref] [PubMed]
- Rydén I, Thurin E, Carstam L, et al. Psychotropic and anti-epileptic drug use, before and after surgery, among patients with low-grade glioma: a nationwide matched cohort study. BMC Cancer 2021;21:248. [Crossref] [PubMed]
- De Witt Hamer PC, De Witt Hamer PC, Klein M, et al. Functional Outcomes and Health-Related Quality of Life Following Glioma Surgery. Neurosurgery 2021;88:720-32. [Crossref] [PubMed]
- DeSisto J, Lucas JT Jr, Xu K, et al. Comprehensive molecular characterization of pediatric radiation-induced high-grade glioma. Nat Commun 2021;12:5531. [Crossref] [PubMed]
- Han S, Yang Z, Wang L, et al. Postoperative hydrocephalus is a high-risk lethal factor for patients with low-grade optic pathway glioma. Br J Neurosurg 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Kavouridis VK, Calvachi P, Cho CH, et al. Patterns of Interaction Between Diffuse Low-Grade Glioma and Pregnancy: An Institutional Case Series. World Neurosurg 2021;150:e236-52. [Crossref] [PubMed]
- Duffau H. Diffuse low-grade glioma, oncological outcome and quality of life: a surgical perspective. Curr Opin Oncol 2018;30:383-9. [Crossref] [PubMed]
- Faulkner H, Arnaout O, Hoshide R, et al. The Surgical Resection of Brainstem Glioma: Outcomes and Prognostic Factors. World Neurosurg 2021;146:e639-50. [Crossref] [PubMed]
- Pitskhelauri DI, Bykanov AE. Complication avoidance: resection of the insular glioma complicated by iatrogenic injury to the lenticulostriate artery. Acta Neurochir (Wien) 2021;163:3093-6. [Crossref] [PubMed]
- García Vicente AM, Rodriguez Muñoz MJ, Pena Pardo FJ, et al. Ischemic Complications After High-Grade Glioma Resection Could Interfere With Residual Tumor Detection With 18F-Fluorocholine PET/CT. Clin Nucl Med 2019;44:e76-84. [Crossref] [PubMed]
- Li J, Wang X, Wang C, et al. The moderating role of depression on the association between posttraumatic growth and health-related quality of life in low-grade glioma patients in China. Psychol Health Med 2019;24:643-53. [Crossref] [PubMed]
- Rahmani M, Hendi K, Ajam H, et al. Alteration of anxiety and depression after awake craniotomy: a prospective study on patients with language eloquent high-grade glioma. J Neurosurg Sci 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Hao A, Huang J, Xu X. Anxiety and depression in glioma patients: prevalence, risk factors, and their correlation with survival. Ir J Med Sci 2021;190:1155-64. [Crossref] [PubMed]
- Song L, Quan X, Su L, et al. Inflammation and behavioral symptoms in preoperational glioma patients: Is depression, anxiety, and cognitive impairment related to markers of systemic inflammation? Brain Behav 2020;10:e01771. [Crossref] [PubMed]
- Wilson AL, McNaughton D, Meyer SB, et al. Understanding the links between resilience and type-2 diabetes self-management: a qualitative study in South Australia. Arch Public Health 2017;75:56. [Crossref] [PubMed]
- Qiu C, Shao D, Yao Y, et al. Self-management and psychological resilience moderate the relationships between symptoms and health-related quality of life among patients with hypertension in China. Qual Life Res 2019;28:2585-95. [Crossref] [PubMed]
- Tamura S. Factors Related to Resilience, Anxiety/Depression, and Quality of Life in Patients with Colorectal Cancer Undergoing Chemotherapy in Japan. Asia Pac J Oncol Nurs 2021;8:393-402. [Crossref] [PubMed]
- Pérez-Aranda A, García-Campayo J, Gude F, et al. Impact of mindfulness and self-compassion on anxiety and depression: The mediating role of resilience. Int J Clin Health Psychol 2021;21:100229. [Crossref] [PubMed]
- Zou G, Li Y, Xu R, et al. Resilience and positive affect contribute to lower cancer-related fatigue among Chinese patients with gastric cancer. J Clin Nurs 2018;27:e1412-8. [Crossref] [PubMed]
- Aizpurua-Perez I, Perez-Tejada J. Resilience in women with breast cancer: A systematic review. Eur J Oncol Nurs 2020;49:101854. [Crossref] [PubMed]
- Litwin MS. The resilience of men: quality of life after prostate cancer. Nat Rev Urol 2019;16:334-5. [Crossref] [PubMed]
- Wang TJC, Mehta MP. Low-Grade Glioma Radiotherapy Treatment and Trials. Neurosurg Clin N Am 2019;30:111-8. [Crossref] [PubMed]
- Tabor JK, Bonda D, LeMonda BC, et al. Neuropsychological outcomes following supratotal resection for high-grade glioma: a review. J Neurooncol 2021;152:429-37. [Crossref] [PubMed]
- Rahmani R, Tomlinson SB, Santangelo G, et al. Risk factors associated with early adverse outcomes following craniotomy for malignant glioma in older adults. J Geriatr Oncol 2020;11:694-700. [Crossref] [PubMed]
- Teng KX, Price B, Joshi S, et al. Life after surgical resection of a low-grade glioma: A prospective cross-sectional study evaluating health-related quality of life. J Clin Neurosci 2021;88:259-67. [Crossref] [PubMed]
- Leonetti A, Puglisi G, Rossi M, et al. Factors Influencing Mood Disorders and Health Related Quality of Life in Adults With Glioma: A Longitudinal Study. Front Oncol 2021;11:662039. [Crossref] [PubMed]
- Umezaki S, Shinoda Y, Mukasa A, et al. Factors associated with health-related quality of life in patients with glioma: impact of symptoms and implications for rehabilitation. Jpn J Clin Oncol 2020;50:990-8. [Crossref] [PubMed]
- Renovanz M, Hickmann AK, Nadji-Ohl M, et al. Health-related quality of life and distress in elderly vs. younger patients with high-grade glioma-results of a multicenter study. Support Care Cancer 2020;28:5165-75. [Crossref] [PubMed]
- Halkett GK, Lobb EA, Rogers MM, et al. Predictors of distress and poorer quality of life in High Grade Glioma patients. Patient Educ Couns 2015;98:525-32. [Crossref] [PubMed]
- Kim SR, Kim HY, Nho JH, et al. Relationship among symptoms, resilience, post-traumatic growth, and quality of life in patients with glioma. Eur J Oncol Nurs 2020;48:101830. [Crossref] [PubMed]
- Edwards KA, Alschuler KA, Ehde DM, et al. Changes in Resilience Predict Function in Adults With Physical Disabilities: A Longitudinal Study. Arch Phys Med Rehabil 2017;98:329-36. [Crossref] [PubMed]
- Ye ZJ, Qiu HZ, Li PF, et al. Predicting changes in quality of life and emotional distress in Chinese patients with lung, gastric, and colon-rectal cancer diagnoses: the role of psychological resilience. Psychooncology 2017;26:829-35. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



