
Role of season in overall survival of patients with bone metastasis undergoing radiotherapy
Despite training for early diagnosis of cancer, advances in the diagnosis and the treatment, bone metastases (BM) are one of the most common problems in clinical follow-up. While bone metastasis is an important prognostic factor in many tumors, early detection and optimal treatment are essential to achieve increased overall-survival and better quality of life (1,2).
The skeletal system is the third most common site in which metastasis occurs after lung and liver (1). Distribution and frequency of bone metastasis vary depending on the method used for diagnosis. The most common cancers; breast, prostate and lung account for 80% of BM. While the incidence of BM in breast cancer patients in the advanced stages is 85%, it is 50–70% for prostate cancer, 32–40% for lung cancer. Seventy percent of the BM involve axial skeleton (cranium, ribs and vertebrae) and 30% involve long bones (2-4). BM mostly occur as a result of hematogenous spread of cancer cells. Micro-metastatic tumor cells are detected in the bone marrow of cancer patients at a rate of 25–75%. But not all of these cells cause clinically evident BM by disrupting the structural integrity of bone. Bone metastasis is a multistep process involving complex interactions. In the presence of tumor cells, balance between bone formation and resorption is disturbed and new bone formation can’t compensate the loss (1,5).
For lung, breast and prostate cancer patients with BM, there is no chance of getting cured with today’s treatment approaches. Therefore, the main goal of the treatment is to improve survival and extend the overall well-being till the progression of disease (1-3). Both systemic and local treatment options are available for the treatment of BM. The choice of treatment depends on patient’s performance, prognosis of the disease and life expectancy (2). In 65–75% of the patients with bone metastasis, there are bone pain and movement disorders. Therefore, the most important goals of the treatment are pain control and preservation of functionality. Radiotherapy is the best treatment option that can be selected in metastatic bone pain. Other treatment options are not as effective as RT in this situation. Radiation therapy technique, dose prescription and fractions are determined according to general condition, life expectancy, mobility, fracture risk assets, metastasis location and number, bone marrow function level and previous treatments of patient. Different radiation regimens are used such as 10×3, 5×4, 1×10 and 1×8 Gy (6-8).
Although there are many published reports regarding important prognostic factors in bone metastasis such as gender and performance status of the patient, primary tumor and its histology, localization of bone metastasis (e.g., weight-bearing) and its type (e.g., osteolytic, osteoblastic), extent of metastasis, number of painful metastases, degree of pain and marital status of the patients, there isn’t a study that investigate whether season is an important factor in the prognosis of bone metastasis (3-5). The aim of this retrospective study was to investigate if season has prognostic importance in cancer patients with bone metastasis.
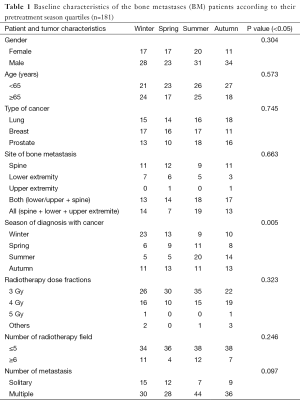
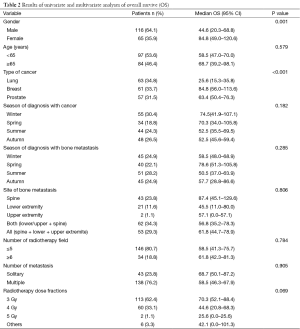
We enrolled 181 patients with evident bone metastasis in our study. Of all the patients, 116 were male and 65 were female with a mean age of 62.8 years old (range, 28–85 years old). In 63 patients (34.8%) primary tumor site was lung, in 61 patients (33.7%) breast, and in 57 patients (31.5%) it was prostate. The mean follow-up time was 10 day–169 months. The mean time for development of bone metastasis was 16.8±3.8 months. The median overall-survival was 22.1 months. While most of the cancer cases were diagnosed during winter, bone metastasis was mostly detected during summer. When the detection of bone metastasis was evaluated according to seasonal distribution, significant result was detected for only cancer type (P<0.001). But for gender, age, type of cancer, location of bone metastasis, number of metastases, number of patients having radiotherapy and radiation fraction dose, there weren’t statistically significant results (Table 1). When the overall-survival was evaluated according to the season of diagnosis, the highest survival was detected in the spring group (78.6 months), and the lowest in the summer group (50.5 months) (P=0.285). In addition, overall-survival of patients who were diagnosed in winter was found to be higher than other seasons (P=0.182). In the univariate and multivariate analysis, factors that affect overall-survival significantly were type of cancer and gender (P<0.001) (Table 2).
Full table
Full table
In our study, BM occurred mostly in lung cancer followed by breast and prostate cancer. Most common site for bone metastasis was vertebrae and then pelvis. Also multiple BM were more frequent. In the literature, cancers that develop bone metastasis frequently appear to be breast, prostate and lung cancer, respectively. In the study of Tubiana-Hulin et al., anatomical distribution of BM was as follows; 59% vertebrae, 49% pelvis, 30% rib, 24% femur, 20% cranium and 13% humerus (1). Tiwana et al. studied 1,880 patients with BM and they found that most common site for metastasis was spine (68.5%) followed by extremity (15.2%) and pelvis (14.4%) (8). In a series of 836 patients (33.2% breast cancer, 24.6% lung cancer), in 285 patients extremity metastases and in 43 patients solitary extremity lesion was detected and the most common part of extremity for bone metastasis was the proximal femur (65.2%) and then humerus (25.6%) (9). In another report, solitary bone lesion was found in 41% of 703 breast cancer patients with metastatic bone lesion and multiple BM was reported in 59% (10). Rates and distribution of metastasis in this study correspond with results of those 703 patients.
We found that overall-survival of lung cancer was shorter than of breast and prostate cancer (P<0.001). In addition, overall-survival was found shorter in male patients, patients with multiple BM, patients who were diagnosed with BM in summer, lower extremity metastases and in patients with multiple metastases. When we compared treatment schemes, we couldn’t find significant difference between fraction doses. In the literature, while overall-survival of lung cancer is measured with months, course of breast and prostate cancer patients with BM is slower and life expectancy varies from 2 to 3 years. Presence of BM shortens long-term survival significantly in lung cancer patients. According to the studies of breast cancer patients in the literature, expected overall-survival in patients with isolated BM was 5–6 years and in the advanced stages with addition of other metastases, survival was reported to be 2 years and in 1–20% of these cases, up to 5 years of overall-survival was observed (3-5,8,9). In the meta-analysis of Wu et al., different treatment schemes in the management of BM were compared and there was no difference between radiotherapy regimens (6).
We found that overall-survival of patients who were diagnosed with cancer in winter and cancer patients who were diagnosed with bone metastasis in spring had higher overall-survival. Robsahm et al. reported that patients who were diagnosed with cancer in winter had a higher risk of death (11). In another work-up, times of diagnosis of lung and breast cancer patients were grouped according to the season and short-term (1 month) and long-term (>5 years) survival were compared. In breast cancer short-term survival was higher in the summer group and in lung cancer long-term survival was higher in the spring group (12).
In conclusion, our initial study indicated that season has no impact on prognosis of cancer patients with bone metastasis. However, this study must be supported by further studies that involve patients from different cultural origins.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2016.01.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tubiana-Hulin M. Incidence, prevalence and distribution of bone metastases. Bone 1991;12:S9-10. [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [PubMed]
- Ulas A, Bilici A, Durnali A, et al. Risk factors for skeletal-related events (SREs) and factors affecting SRE-free survival for nonsmall cell lung cancer patients with bone metastases. Tumour Biol 2015; [Epub ahead of print]. [PubMed]
- Nørgaard M, Jensen AØ, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 2010;184:162-7. [PubMed]
- Yong M, Jensen AÖ, Jacobsen JB, et al. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999-2007). Breast Cancer Res Treat 2011;129:495-503. [PubMed]
- Wu JS, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 2003;55:594-605. [PubMed]
- Wong E, Hoskin P, Bedard G, et al. Re-irradiation for painful bone metastases - a systematic review. Radiother Oncol 2014;110:61-70. [PubMed]
- Tiwana MS, Barnes M, Yurkowski E, et al. Incidence and treatment patterns of complicated bone metastases in a population-based radiotherapy program. Radiother Oncol 2015; [Epub ahead of print]. [PubMed]
- Fukuma H, Beppu Y, Chuma H, et al. Diagnosis and treatment of metastatic bone cancer of the extremities. Gan To Kagaku Ryoho 1987;14:1729-38. [PubMed]
- Koizumi M, Yoshimoto M, Kasumi F, et al. Comparison between solitary and multiple skeletal metastatic lesions of breast cancer patients. Ann Oncol 2003;14:1234-40. [PubMed]
- Robsahm TE, Tretli S, Dahlback A, et al. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control 2004;15:149-58. [PubMed]
- Roychoudhuri R, Robinson D, Coupland V, et al. Season of cancer diagnosis exerts distinct effects upon short- and long-term survival. Int J Cancer 2009;124:2436-41. [PubMed]


