
Increase of CD3+CD7− T cells in bone marrow predicts invasion in patients with T-cell non-Hodgkin’s lymphoma
Introduction
T-cell lymphoma (TCL) is a malignant clonal proliferative disease originating from T lymphocytes, accounting for 10–15% of non-Hodgkin’s lymphoma (NHL) (1). It is a malignant cancer with rapid progress, poor prognosis and high recurrence rate. The 5-year survival rate is about 30–40% (2). Patients with bone marrow (BM) invasion are in stage IV according to the Ann Arbor staging. Although T-NHL has a lower BM invasion rate compared to B-NHL (3), T-NHL patients with BM invasion have a shorter survival time than B-NHL patients (4). So far, BM biopsy and BM pathology are still the gold standard for diagnosis of BM invasion in T-NHL (5). However, with the technology development, several methods such as positron emission tomography-computed tomography (PET-CT) (6,7), T-cell receptor (TCR) gene rearrangement (3,8), flow cytometry (9-11) have been greatly improved, and gradually become required examinations when the BM invasion of T-NHL was assessed. Although a growing body of evidence manifests that BM biopsy is still an irreplaceable diagnostic method (11) and the current pathological diagnosis has certain limitations, a pathological evaluation is one of the most important methods for the diagnosis of malignant lymphoma with BM invasion (12). The method based on flow cytometry has a high sensitivity, making it easier to detect the minimal BM invasion (9).
CD7 is a cell surface costimulatory molecule expressed on T and natural killer (NK) cells and on cells in the early stages of T-, B-, and myeloid cell differentiation. Interestingly, under physiological and certain pathological conditions, the loss of CD7 molecule occurs in a subset of CD4+ memory T cells. Specific absence of CD7 antigen expression on T cells has been observed in various pathologic conditions such as HIV infection (13), rheumatoid arthritis, and kidney transplantation with the consequence of chronically repeated T cell stimulation (14,15). While loss of CD7 gene expression indicates malignancy in the peripheral blood T cells and has been observed in a great variety of pathologic conditions such as cutaneous T cell lymphoma, adult T-cell leukemia/lymphoma, primary hepatic peripheral T-cell lymphoma, chronic myeloid leukemia, and Sezary syndrome (SS) (16). However, the number and activity of CD7− T cells as well as the potential pathophysiological significance of this T cell subset in the BM have not been discussed. Additionally, the CD7 monoclonal antibodies (17) or CD7 chimeric antigen receptor (CAR)-T cells (18) have exhibited powerful cytotoxic killing effects on CD7+ hematological malignant diseases.
Immune checkpoints include stimulatory and inhibitory checkpoint molecules. Over the past decade, inhibitory checkpoints, including cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death-1 (PD-1), lymphocyte-activation-gene-3 (LAG-3) and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), have been identified to suppress anti-tumor immune responses in solid tumors. Novel drugs targeting immune checkpoints, such as specific PD-1 blockades, have already achieved approval in several solid tumors such as melanoma, non-small cell lung cancer, and kidney cancer (19,20). As for hematological malignancies, clinical benefits of immune checkpoint blockades are observed in only limited tumor types such as Hodgkin’s lymphoma that are particularly characterized by a high infiltration of immune cells. The main reason is primary and acquired resistance that remains a barrier in utilizing them against a broad range of hematological malignancies.
In our study, we found that CD3+CD7− T cells increased significantly in the BM in T-NHL patients with BM invasion. The proportion of CD3+CD7− T cells in nucleated cells in BM was a potential diagnostic predictor of BM invasion in T-NHL. This may be related to increased expression of immune inhibitory checkpoints in these cells. We present the following article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2666/rc).
Methods
Enrolled patients and BM sample
This study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (No. 2017-044), and all participants signed written informed consent. The study was carried out in accordance with the Declaration of Helsinki (as revised in 2013). A total of 85 patients who were newly diagnosed with T-NHL lymphoma consist of 59 males and 26 females with a median age of 60 years old (range, 17–83 years old). The diagnosis and classification depend on pathological examination after lymph node puncture or biopsy and PET-CT. The stages and other clinical characteristics of patients are summarized in Table 1. Bone marrow mononuclear cells (BMMNCs) were isolated using ficoll-hypaque density gradient centrifugation and stored.
Table 1
| Characteristics | CD3+CD7− ≥1.035% (n=24) | CD3+CD7− <1.035% (n=61) | P value |
|---|---|---|---|
| Median age [range] (years) | 63.5 [28–77] | 57 [17–83] | 0.430 |
| Male/female | 15/9 | 44/17 | 0.438 |
| Ann Arbor stage, n (%) | |||
| I | 3 (12.5) | 16 (26.2) | 0.249 |
| II | 3 (12.5) | 11 (18.0) | 0.748 |
| III | 5 (20.8) | 18 (29.5) | 0.589 |
| IV | 13 (54.2) | 15 (24.6) | 0.012 |
| Unclassified | 0 | 1 (1.6) | 1.000 |
| Diagnostic criteria, n (%) | |||
| Pathology | 19 (79.2) | 58 (95.1) | – |
| PET-CT | 20 (83.3) | 57 (93.4) | – |
| Marrow invasion, n (%) | 12 (50.0) | 12 (19.7) | 0.008 |
| Marrow invasion criteria, n (%) | |||
| Pathology | 3 (12.5) | 3 (4.9) | – |
| PET-CT | 1 (4.2) | 2 (3.3) | – |
| Flow cytometry | 12 (50.0) | 8 (13.1) | – |
| Bone marrow smear | 10 (41.7) | 7 (11.5) | – |
| TCR gene rearrangement | 11 (45.8) | 17 (27.9) | – |
PET-CT, positron emission tomography-computed tomography; T-NHL, T-cell non-Hodgkin’s lymphoma; TCR, T-cell receptor.
Immunophenotyping
BMMNCs were stained with different combinations of anti-human CD3 (Beckman Coulter Cat# IM2467, RRID:AB_130788), CD4 (Beckman Coulter Cat# IM2468, RRID:AB_130781), CD7 (Beckman Coulter Cat# IM3613U, RRID:AB_10643230), CD8 (Beckman Coulter Cat# IM0451U, RRID:AB_10638218), CD28 (Beckman Coulter Cat# 6607111, RRID:AB_1575955), CD45 (Beckman Coulter Cat# A96416, RRID:AB_2888654), human leucocyte antigen DR (HLA-DR) (Beckman Coulter Cat# A74781, RRID:AB_2892134) for 20 min in dark and PD-1 (BD Biosciences Cat# 557946, RRID:AB_647199; San Jose, CA, USA), LAG3 (BioLegend Cat# 369310, RRID:AB_2629753; San Diego, CA, USA), C-C motif chemokine receptor 7 (CCR7) for 30 min in dark. Cells were then hemolyzed for 15 min and washed twice. The immunophenotyping was performed using Navios flow cytometer (Beckman Coulter, Brea, CA, USA). Live lymphocytes were gated according to CD45 and appropriate forward and side scatter. Gating of CD3 was set to determine the frequency of CD3+CD7− T cells. The proportion of LAG3, PD-1, CCR7, HLA-DR or CD28 was determined in CD3+CD7− T cells, CD3+CD7+ T cells, CD3+CD7−CD4+ T cells, and CD3+CD7+CD4+ T cells. The gating standards of CD28, HLA-DR, PD-1, CCR7 and LAG3 were set using isotype controls recommended by the manufacturer. A total of 500,000 cells per sample was acquired and analyzed.
Statistical analysis
The data were presented as mean ± standard error of the mean (SEM) and analyzed by t-test, one-way ANOVA or Chi-square test to determine the differences. In this study, the proportion of CD3+CD7− T cells of nucleated cells was measured in all 85 patients, and the receiver operating characteristic (ROC) curve was subsequently used in clinical monitoring to calculate the appropriate values for sensitivity and specificity. Differences at P<0.05 were considered statistically significant, and all tests were two-tailed. All statistical analyses were performed using GraphPad Prism 5.0 software.
Results
Higher frequency of CD3+CD7− T cells is shown in BM in patients with T-NHL BM invasion
All BM samples of 85 patients who were newly diagnosed with T-NHL were analyzed by flow cytometry. All cells in the CD45high/SSClow population were predominantly T-cells with regular CD3 expression. It has been reported that in cutaneous TCLs, especially SS, a large number of circulating CD7− T cells are derived from the clonal expansion of CD7− T cells in tumor tissues (14,16). We found that the frequency of CD3+CD7− T cells in BM was notably higher in T-NHL patients with BM invasion than that patients without BM invasion or healthy donors (Figure 1A-1C). The ratio of CD4/CD8, although high, was not statistically significant between the BM invasion patients and patients without BM invasion or healthy donors (Figure 1D). In this study, BM invasion was defined as positive results in two or more examination including BM biopsy, PET-CT, BM smear, flow cytometry and TCR gene rearrangements.
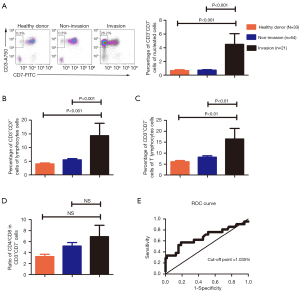
The ROC curve of the proportion of CD3+CD7− T cells of nucleated cells was used to determine the values (Figure 1E). The area under the curve (AUC) of proportion of CD3+CD7− T cells of nucleated cells was 0.6749 [95% confidence interval (CI): 0.5226 to 0.8271], and the optimal value was 1.035%, with 57.1% sensitivity and 81.2% specificity, P=0.017. There were 24 (28.3%) patients with high proportion of CD3+CD7− T cells of nucleated cells (≥1.035%) and 61 patients with low proportion of CD3+CD7− T cells of nucleated cells (<1.035%). The ratio of invasion in high group was markedly higher than that in low one (P=0.0015). The association between proportion of CD3+CD7− T cells of nucleated cells and laboratory indicators was summarized in Table S1. However, there was no significant difference in the overall survival (OS) between those two groups (Figure S1).
Analysis for CD3+CD7− T cells in different subtypes and viral Epstein-Barr virus (EBV)-DNA
T-NHL is a disease with obvious heterogeneity. There are considerable differences in clinical manifestations and immunological phenotypes between different subtypes. 85 patients enrolled, included 18 (21.2%) cases of angioimmunoblastic T-cell lymphoma (AITL), 9 (10.6%) cases of peripheral T-cell lymphoma (PTCL), 9 (10.6%) cases of anaplastic large cell lymphoma (ALCL), 13 (15.3%) cases of peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 (7.1%) cases of T cell lymphoblastic lymphoma (TLBL), and 30 (35.3%) cases of natural killer/T-cell lymphoma (NKTCL). There was no difference in the proportion of CD3+CD7− T cells of nucleated cells among different types (Figure 2A).
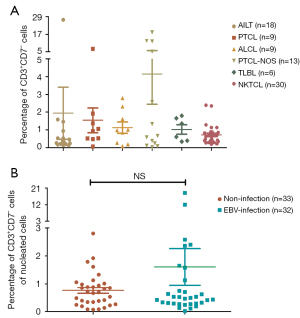
EBV infection is closely related to the occurrence of T cell lymphoma. A total of 65 cases in our sample were tested for the level of EBV-DNA in peripheral blood, of which 32 cases were positive. No difference was observed in the proportion of CD3+CD7− T cells in nucleated cells between two groups (Figure 2B).
CD3+CD7− T cells express higher level of immune inhibitory checkpoints
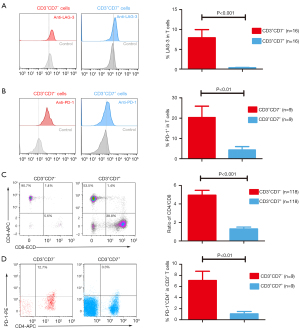
In order to investigate the mechanism by which CD3+CD7− T cells may cause BM invasion, the expression of immune markers on these cells were analyzed. Compared with CD3+CD7+ T cells, the expression of immune checkpoints like LAG3 and PD-1 was higher in CD3+CD7− T cells (Figures 3A,3B), but there was no significant difference on the expression of CCR7, CD28 and HLA-DR (Figure S2). Furthermore, we found that these CD3+CD7− T cells had a higher ratio of CD4/CD8 (Figure 3C). Additionally, we also found that the expression of PD-1 was higher in CD3+CD7−CD4+ T cells than that in CD3+CD7+CD4+ T cells (Figure 3D). These data suggested that CD3+CD7− T cells may be more exhausted compared to CD3+CD7+ T cells.
Discussion
CD7− T cells are usually considered as late memory cells characterized by a high activation threshold, low effector capacities, and high sensitivity to activation-induced cell death (AICD) (14,21).The CD7− T cells can be found in peripheral blood, especially in patients with SS or infection. Viruses infection including EBV and cytomegalovirus (CMV), or the immunization with attenuated measles virus, may provoke a clonal but benign expansion of CD3+CD7− T cells (22). In acute myeloid leukemia (AML), CD7 had already been confirmed as an independent prognostic factor for poor disease-free survival and post-remission survival (23). In lymphoma, especially T-NHL, CD7 has become one of the therapeutic targets (18). However, the pathophysiological significance of CD3+CD7− T cells in the BM remains elusive in T-NHL.
Here, we found that the proportion of CD3+CD7− T cells was especially higher in T-NHL patients with BM invasion. We divided all patients into two subgroups according to ROC curve, and the results showed that patients with a high proportion of CD3+CD7− T cells of nucleated cells had a higher BM invasion rate than those with a low level. However, in T-NHL patients, EBV infection did not cause a change in the proportion of CD3+CD7− T cells, which may be due to its own T cell function impairment in T-NHL patients, especially in BM-invasion patients.
CD28, a cell surface glycoprotein receptor, which has sequence homology with CTLA-4 and is expressed on T cells after activation, regulates T cell proliferation and differentiation and plays an important role in the immune response pathway in vivo (24). HLA-DR exists on the surface of macrophages, B lymphocytes, and T lymphocytes. In addition to being involved in antigen presentation, HLA-DR is also recognized as a marker of T cell activation. PD-1, a well-recognized immune checkpoint, has been extensively studied in hematological malignancies (25-27). The application of PD-1 inhibitors has received approval in Hodgkin’s lymphoma (28). LAG3 is a key immune checkpoint with relevance in autoimmunity, cancer and infectious diseases (29). In this study, we found that CD3+CD7− T cells had higher expression of PD-1 and LAG3 compared with CD3+CD7+ T cells. Since LAG3 and PD-1 contribute to T-cell exhaustion during chronic virus infection (30), high expression of these two immune checkpoints on CD3+CD7− T cells indicated that these cells were exhausted during the immune response against T-NHL. The CD4/CD8 ratio in normal BM generally fluctuates within a certain relatively fixed range. We found a significantly higher ratio of CD4/CD8 in CD3+CD7− T cells, which may be also related to immune dysfunction.
Except for targeted therapy, the CAR-T cell therapy has been widely used in B-cell lymphoma and multiple myeloma. However, it is just beginning in T-cell hematological malignancies. CD7 CAR-T cells exhibit a powerful cytotoxic killing effect on CD7+ hematological malignancies (18). In this study, increased CD7− T cells were observed in the BM of T-NHL patients with BM invasion. Therefore, we think that it is necessary to fully evaluate the proportion of CD7− T cells in the BM before selecting CAR-T treatment.
Of course, we must admit that our retrospective analysis certainly has limitations. As recruitment was performed in a single institution, election bias might be difficult to be well balanced. Secondly, because of the limited follow-up time, we drew OS curve under the grouping of ROC curves, and there was no significant difference between two groups, which may due to the limiting time and cases, we will finish it in our subsequent studies. Additionally, although we found that the expression of immune inhibitory checkpoints increased in CD3+CD7− T cells, the specific mechanism need be further studied.
In conclusion, we believe that the frequency of CD3+CD7− T cells is significantly increased in T-NHL with BM invasion, and the proportion of CD3+CD7− T cells of nucleated cells in BM was a potential diagnostic predictor of BM invasion in T-NHL. In addition, increased expression of several immune inhibitory checkpoints was found on CD3+CD7− T cells, which can guide clinical diagnosis and medication to a certain extent. This study supplies an auxiliary method which may indicate that some indolent T lymphomas are in a progressive state or may indicate a poor prognosis.
Acknowledgments
Funding: This work was supported by Zhejiang Provincial Natural Science Foundation of China (No. LY20H080005), National Natural Science Foundation of China (No. 82072354), and the Science and Technology Project from Health Commission of Zhejiang Province (No. 2020KY179).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2666/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2666/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2666/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (No. 2017-044), and all participants signed written informed consent. The study was carried out in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sheikhpour R, Pourhosseini F, Neamatzadeh H, et al. Immunophenotype evaluation of Non-Hodgkin's lymphomas. Med J Islam Repub Iran 2017;31:121. [Crossref] [PubMed]
- Busemann C, Klein S, Schmidt CA, et al. Treatment of High-Risk T-NHL with Stem Cell Transplantation: A Single Center Experience. Indian J Hematol Blood Transfus 2015;31:14-20. [Crossref] [PubMed]
- Kang YH, Park CJ, Seo EJ, et al. Polymerase chain reaction-based diagnosis of bone marrow involvement in 170 cases of non-Hodgkin lymphoma. Cancer 2002;94:3073-82. [Crossref] [PubMed]
- Conlan MG, Bast M, Armitage JO, et al. Bone marrow involvement by non-Hodgkin's lymphoma: the clinical significance of morphologic discordance between the lymph node and bone marrow. Nebraska Lymphoma Study Group. J Clin Oncol 1990;8:1163-72. [Crossref] [PubMed]
- Schwonzen M, Pohl C, Steinmetz T, et al. Bone marrow involvement in non-Hodgkin's lymphoma: increased diagnostic sensitivity by combination of immunocytology, cytomorphology and trephine histology. Br J Haematol 1992;81:362-9. [Crossref] [PubMed]
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014;32:3048-58. [Crossref] [PubMed]
- Chen S, Wang S, He K, et al. PET/CT predicts bone marrow involvement in paediatric non-Hodgkin lymphoma and may preclude the need for bone marrow biopsy in selected patients. Eur Radiol 2018;28:2942-50. [Crossref] [PubMed]
- Rosolen A, Perkins SL, Pinkerton CR, et al. Revised International Pediatric Non-Hodgkin Lymphoma Staging System. J Clin Oncol 2015;33:2112-8. [Crossref] [PubMed]
- Gomyo H, Shimoyama M, Minagawa K, et al. Morphologic, flow cytometric and cytogenetic evaluation of bone marrow involvement in B-cell lymphoma. Haematologica 2003;88:1358-65. [PubMed]
- Kaleem Z. Flow cytometric analysis of lymphomas: current status and usefulness. Arch Pathol Lab Med 2006;130:1850-8. [Crossref] [PubMed]
- Kim B, Lee ST, Kim HJ, et al. Bone marrow flow cytometry in staging of patients with B-cell non-Hodgkin lymphoma. Ann Lab Med 2015;35:187-93. [Crossref] [PubMed]
- Cabezas-Quintario MA, Gomez P, Yuste-Del Pozo V, et al. Bone marrow trephine biopsy involvement by lymphoma: pattern of involvement and concordance with flow cytometry, in 10 years from a single institution. Clin Transl Oncol 2016;18:537-40. [Crossref] [PubMed]
- Wallace DL, Matear PM, Davies DC, et al. CD7 expression distinguishes subsets of CD4(+) T cells with distinct functional properties and ability to support replication of HIV-1. Eur J Immunol 2000;30:577-85. [Crossref] [PubMed]
- Reinhold U, Abken H. CD4+ CD7- T cells: a separate subpopulation of memory T cells? J Clin Immunol 1997;17:265-71. [Crossref] [PubMed]
- Rappl G, Schrama D, Hombach A, et al. CD7(-) T cells are late memory cells generated from CD7(+) T cells. Rejuvenation Res 2008;11:543-56. [Crossref] [PubMed]
- Rappl G, Muche JM, Abken H, et al. CD4(+)CD7(-) T cells compose the dominant T-cell clone in the peripheral blood of patients with Sézary syndrome. J Am Acad Dermatol 2001;44:456-61. [Crossref] [PubMed]
- Yu Y, Li J, Zhu X, et al. Humanized CD7 nanobody-based immunotoxins exhibit promising anti-T-cell acute lymphoblastic leukemia potential. Int J Nanomedicine 2017;12:1969-83. [Crossref] [PubMed]
- Gomes-Silva D, Srinivasan M, Sharma S, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 2017;130:285-96. [Crossref] [PubMed]
- Ribeiro Gomes J, Schmerling RA, Haddad CK, et al. Analysis of the Abscopal Effect With Anti-PD1 Therapy in Patients With Metastatic Solid Tumors. J Immunother 2016;39:367-72. [Crossref] [PubMed]
- Kreft S, Gesierich A, Eigentler T, et al. Efficacy of PD-1-based immunotherapy after radiologic progression on targeted therapy in stage IV melanoma. Eur J Cancer 2019;116:207-15. [Crossref] [PubMed]
- Sempowski GD, Lee DM, Kaufman RE, et al. Structure and function of the CD7 molecule. Crit Rev Immunol 1999;19:331-48. [PubMed]
- Klameth A, Neubauer A, Keller C, et al. Aberrant CD3-Positive, CD8-Low, CD7-Negative Lymphocytes May Appear During Viral Infections and Mimic Peripheral T-Cell Lymphoma. Diagnostics (Basel) 2020;10:204. [Crossref] [PubMed]
- Chang H, Yeung J, Brandwein J, et al. CD7 expression predicts poor disease free survival and post-remission survival in patients with acute myeloid leukemia and normal karyotype. Leuk Res 2007;31:157-62. [Crossref] [PubMed]
- Xia S, Chen Q, Niu B. CD28: A New Drug Target for Immune Disease. Curr Drug Targets 2020;21:589-98. [Crossref] [PubMed]
- Nguyen GH, Olson LC, Magro CM. Upregulation of inhibitory signaling receptor programmed death marker-1 (PD-1) in disease evolution from cutaneous lymphoid dyscrasias to mycosis fungoides and Sezary's syndrome. Ann Diagn Pathol 2017;28:54-9. [Crossref] [PubMed]
- Zaja F, Tabanelli V, Agostinelli C, et al. CD38, BCL-2, PD-1, and PD-1L expression in nodal peripheral T-cell lymphoma: Possible biomarkers for novel targeted therapies? Am J Hematol 2017;92:E1-2. [Crossref] [PubMed]
- Dong Y, Han Y, Huang Y, et al. PD-L1 Is Expressed and Promotes the Expansion of Regulatory T Cells in Acute Myeloid Leukemia. Front Immunol 2020;11:1710. [Crossref] [PubMed]
- Wang C, Liu Y, Dong L, et al. Efficacy of Decitabine plus Anti-PD-1 Camrelizumab in Patients with Hodgkin Lymphoma Who Progressed or Relapsed after PD-1 Blockade Monotherapy. Clin Cancer Res 2021;27:2782-91. [Crossref] [PubMed]
- Graydon CG, Mohideen S, Fowke KR. LAG3's Enigmatic Mechanism of Action. Front Immunol 2021;11:615317. [Crossref] [PubMed]
- Turnis ME, Andrews LP, Vignali DA. Inhibitory receptors as targets for cancer immunotherapy. Eur J Immunol 2015;45:1892-905. [Crossref] [PubMed]


