
CD73 promotes cervical cancer growth via EGFR/AKT1 pathway
Introduction
CD73 is a 70 kd, glycosylphosphatidylinositol (GPI) anchored cell surface protein, also known as ecto-5'-nucleotidase (ecto-5'NT, EC 3.1.3.5), which is encoded by NT5E gene (Gene ID: 4907) (1). CD73 is high expressed in various types of tumor cells, which has been found to be correlation with tumor cell's proliferation, adhesion, migration and invasion (2-6). And moreover, multiple retrospective studies have showed that CD73 overexpression is correlated with clinical stages, metastasis, prognosis of cancer patients (7-13). In preclinical experiments, CD73-blocked have showed the anti-tumor effects (14-16). CD73 has both enzymatic and non-enzymatic functions. As an enzyme, CD73 catalyzes the hydrolysis of AMP into adenosine and phosphate (17). In addition to its enzymatic function, CD73 have been found to be a regulatory molecule which related to tumor cell’s proliferation and metastatic properties, while the mechanism is unclear (18). In our previous studies, we found that CD73 overexpression might be correlated with EGFR/AKT1 signal pathway in cervical cancer cells (3). In this present study, we further verified the functions and possible mechanism of EGFR and AKT1 in cervical cancer cells with higher CD73 expression level. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2446/rc).
Methods
Cell culture
Human cervical cancer cell lines Hela (RRID:CVCL_0030) and SiHa (RRID:CVCL_0032) was preserved by clinical Laboratory (Tangdu Hospital, Air Force Military Medical University, China). The cells were cultured in Dulbecco’s modified eagle medium (DMEM, Gibco, Carlsbad, NY, USA) medium plus 10% heat-inactivated fetal bovine serum (FBS, SijiqingBiotec, Hangzhou, China) at 37 ℃ with 5% CO2 in a humidified incubator.
CD73 lentivirus packaging and stable cell models construction
CD73 encoding gene sequence was cloned by Polymerase chain reaction (PCR). Then, CD73 lentivirus plasmid were constructed by ligating cloned fragment to lentivirus vector (pLenti-C-Myc-DDK-IRES-Puro). The CD73 lentivirus plasmid and two helper plasmids (PAX2 and VSVG) were transfected into HEK293T cells, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Cell supernatant were collected and concentrated after 72 h. The recombinant lentivirus was stored at −80 ℃, named CD73/Lentivirus. Hela and SiHa cells were infected with CD73/Lentivirus. Stable cell models were screened with 5 µg/mL puromycin.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR (qRT-PCR) was carried out using FastStart Essential DNA Green Master kit (Roche) and was used to detect the mRNA expression levels. The primer sequences were list in Table 1.
Table 1
| Gene | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| CD73 | GCCTGGGAGCTTACGATTTTG | TAGTGCCCTGGTACTGGTCG |
| EGFR | CCAAGGCACGAGTAACAA | ACATAACCAGCCACCTCC |
| AKT1 | ACTGTCATCGAACGCACCTT | TTCTGCAGGACGCGGTTCTC |
| CDK1 | AGCTTTTGGAATACCTATCAG | AAAAGTGGTTTCTTAGTTGCT |
| CDK2 | GCCAGAAACAAGTTGACGG | ATGAGGGGAAGAGGAATGCCAGT |
| CDK3 | TAAGTGGACCAGGAAGGGAC | GGGCAGTCTTGGCTGTGAT |
| CDK4 | CGACCAGTTGGGCAAAATC | CCCGACTCCTCCATCTCAG |
| CDK6 | CCAGCAGCGGACAAATAAA | CTGCAAATATGCAGCCAACACT |
| CDK7 | TAGGGATCTGAAACCAAACA | ACCATACATCCTAGCTCCAA |
| CDKN1A | GGGGACAGCAGAGGAAGAC | CGGCGTTTGGAGTGGTAG |
| CDKN1B | GGGGCTCCGGCTAACTCTGA | GCAGGTCGCTTCCTTATTCC |
| GAPDH | GGTGGTCTCCTCTGACTTCAACAG | GTTGCTGTAGCCAAATTCGTTGT |
Flow cytometry (FCM)
FCM were used to evaluate the CD73 expression. In brief, Cells were obtained and resuspended in PBS buffer at a concentration of 1×105 cells/mL, and then incubated with fluorescein isothiocyanate (FITC)-labeled anti-CD73 antibody (CD73/FITC) for 30 min at 37 ℃. Then cells were washed twice with PBS. Following that, cells were analyzed using FACS calibur flow cytometer (BD Biosciences, San Jose, USA).
Small interfering RNA (siRNA) transfection
EGFR- and AKT1-targeted siRNA and irrelevant control siRNA (NC) were synthesized by Sangon Biotech (Sangon Biotech, Shanghai, China). The siRNA transfection was performed using Lipofectamine 2000. siRNA sequences were list in Table 2.
Table 2
| Name | Sense | Antisense |
|---|---|---|
| si-EGFR | GCAGAGGAAUUAUGAUCUUTT | AAGAUCAUAAUUCCUCUGCTT |
| si-AKT1 | AGGAAGUCAUCGUGGCCAATT | UUGGCCACGAUGACUUCCUTT |
| NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
Cell proliferation assay
Cell proliferation were detected by using a xCELLigence system (RTCA Analyzer DP version, ACEA Biosciences). In short, 1×104 cells in each well were used for RTCA assay. First, 50 µL DMEM with 10% FBS were added in each well of E-plate for baseline determination. After baseline determination, 1×104 cells in DMEM with 10% FBS were added in E-plate wells, then, E-plate was placed in the RTCA analyzer at 37 ℃ in a 5% CO2 humidified incubator. Impedance measurements at every 5 min for 72–96 h. Data of cell proliferation were analyzed via RTCA-soft (ACEA Biosciences).
Cell cycle analysis
Cell cycle was analyzed by ethanol-fixed cells stained with propidium iodide (PI) in buffer containing RNase A. The DNA content was assessed by using FACS calibur flow cytometer (BD Biosciences). Cell cycle analysis were performed 48 h after siRNA transfection.
Cell apoptosis analysis
Cell apoptosis was detected using the annexin V-FITC apoptosis detection kit (KeyGENBioTECH, Nanjing, China). Briefly, cells were collected and washed with PBS twice, stained for 15 min at room temperature with annexin V-FITC and PI. Following that, cell apoptosis was examined using FACS calibur flow cytometer (BD Biosciences). The percentage of apoptotic cells was calculated with CellQuest pro. Cell apoptosis analysis was performed 48 h after siRNA transfection. Statistical analysis of late apoptotic cells double-stained with annexin V-FITC and PI.
Animal preparation
Twenty 6-week-old female BALB/c nude mice (RRID: IMSR_JCL: JCL: mID-0001) were purchased and feed by animal Center of Air Force Military Medical University for tumor xenogenesis experiment. The mice were raised in groups under controlled conditions, each group were kept in individually ventilated cages at a temperature of 20 to 24 ℃, humidity of 50% to 60%, and a 12 h light/dark cycle, with ad libitum access to mice chow.
Murine xenograft models
The effect of CD73 overexpression on tumor growth in vivo were assessed by subcutaneous injection of 4×106 Hela cells infected with CD73/Lentivirus or control lentivirus into BALB/c nude mouse. Twenty mice were randomly assigned to four groups, five female mice were in each group (age: 6–7 weeks; body weight: 20–25 g). The tumors grow to approximately 40 mm3 to initiate the tests, tumors size were measured every 5 days with a vernier caliper for 25 days. The maximum size the tumors allowed to grow was 2,000 mm3, The mice were sacrificed by neck dislocation, and the mice were confirmed dead without activity. The volume of tumor was calculated according to the formula: a×b2×0.5 (a, largest diameter; b, perpendicular diameter). All procedure of animal experiments was processed according to animal welfare guidelines approved by Air Force Medical University’s Institutional Animal Care and Use Committee.
Statistical analysis
All data were confirmed in three biological replicates. Data were calculated and presented as the mean ± SD. ANOVA and Tukey test were used to perform comparisons of multiple groups. Comparison between two groups were conducted by using the Student’s t-test. P<0.05 was considered to be statistical significant. All analysis was performed with R software (version 3.4.2).
Ethical statement
Experiments were performed under a project license (No. 20200505) granted by Air Force Medical University’s Institutional Animal Care and Use Committee, in compliance with Air Force Medical University’s guidelines for the care and use of animals.
Results
Overexpression of CD73 promote cervical cancer cells proliferation in vitro and in vivo
Hela and SiHa cells were infected with CD73/lentivirus or negative control lentivirus, followed by screened with puromycin. The stable cell models with CD73 overexpression were named HelaCD73 and SiHaCD73. Compared with control cells (HelaNC and SiHaNC), the expression levels of CD73 were significantly increased in HelaCD73 and SiHaCD73 cells (Figure 1A-1D). Then the effect of CD73 overexpression on cervical cancer cells proliferation was examined. RTCA results showed that the proliferation rate of HelaCD73 and SiHaCD73 cells were higher than control cells (Figure 1E,1F). Following, HelaCD73 and HelaNC cells were injected subcutaneously into athymic nude mice to construct murine xenograft models. The results showed that tumor growth rate was significantly faster in CD73 overexpressed xenograft models than that in control group (Figure 1G,1H). Taken together, CD73 overexpression could promote cervical cancer cells proliferation in vitro and tumor growth in vivo.
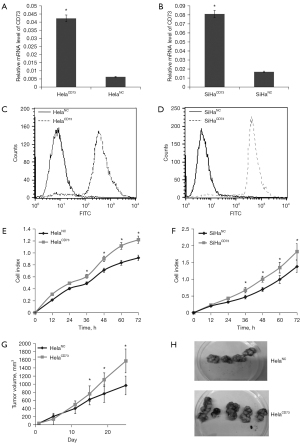
EGFR and AKT1 play important role in growth of HelaCD73 and SiHaCD73 cells
Previous study has shown that CD73 overexpression was positive correlation with EGFR and AKT1 expression. Consistently, in this study, we found that the expression levels of EGFR and AKT1 were increased, both in CD73 overexpressed cancer cell models and transplanted tumor tissues (Figure 2A-2C). Furthermore, to verify the function of EGFR and AKT1 expression level on proliferation of CD73 overexpressed cervical cancer cells, the transient transfection of si-EGFR and si-AKT1 was used to suppress EGFR or AKT1 expression in HelaCD73 and SiHaCD73 cells (Figure 2D,2E). RTCA experiments showed that knockdown of EGFR and AKT1 could significantly decrease the proliferation rate of HelaCD73 and SiHaCD73 cells (Figure 2F,2G). The results suggested the important roles of EGFR and AKT1 signal in increased proliferation rate of CD73 overexpressed cervical cancer cells.
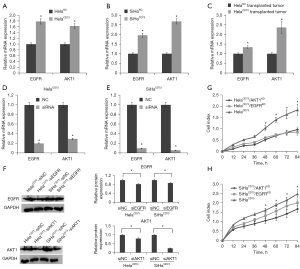
Interference of EGFR and AKT1affects cell cycles and apoptosis of CD73 overexpressing cells
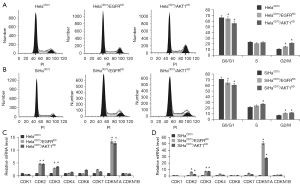
To understand the potential mechanism of EGFR and AKT1 in promoting proliferation of CD73 overexpressed cervical cancer cells. We investigated the effects of EGFR and AKT1 on cell cycle and apoptosis of HelaCD73 and SiHaCD73 cells. First, the results showed that knockdown of EGFR and AKT1 leaded to cell cycle reduced in G1 phase and increased in G2/M of HelaCD73 and SiHaCD73 cells (Figure 3A,3B). And moreover, we investigated the expression levels of cell cycle related molecules. The results showed that EGFR and AKT1 knockdown significantly promote expression level of CDK2, CDK3 and CDKN1A (Figure 3C,3D). Second, FCM results showed that knockdown of EGFR and AKT1could promote HelaCD73 and SiHaCD73cells apoptosis (Figure 4A,4B). Compared with control cells, the apoptosis rate of EGFR and AKT1 knockdown cells increased 2–3 times (P<0.05). Taken together, these results demonstrated that knockdown of EGFR and AKT1 inhibited HelaCD73 and SiHaCD73 cells proliferation via inducing of cell cycles arrest and cell apoptosis.

Discussion
CD73 is widely expressed on the surface of various tissues and cells in the human body, including lymphocytes, endothelial cells, and epithelial cells. CD73 is involved in multiple biological progress such as immune regulation, cell adhesion, angiogenesis (19,20). CD73 have been found to be a key regulatory molecule in development of cancer, and is overexpression in a variety of cancer cells and patients’ biopsies (21). In our studies, we showed that CD73 overexpression promoted cervical cancer cells proliferation in vitro and tumor growth in vivo.
EGFR/Akt signal play important roles in tumor cell’s biological progression, such as proliferation, invasion, apoptosis, etc. Thus, EGFR/Akt signal has been showed to be involved in development of various tumor types, including cervical cancer. Anti-EGFR therapy has become an effective treatment or for several cancer types (22-24). Accumulating data have showed that HPV actives EGFR/Akt signaling in cervical cancer (25-29). EGFR is present in normal and cervical cancer tumors and is expressed to varying degrees, making cervical cancer more accessible to targeted therapy. The expression correlation between EGFR and CD73 have been found in several cancer types. For example, Shali et al. have reported that CD73 and EGFR expression are positively correlated in liver cancer tissue samples (30). Wu et al. have found that CD73 overexpression in colon cancer cell lines can up-regulated EGFR (31). Our data showed that expression levels of EGFR and AKT1 were increased in cancer cells with higher CD73 levels, and also in transplanted tumor tissues. These results suggested that EGFR and AKT1 played important role in increased growth rate of CD73 overexpression cancer cells. This hypothesis was validation by using EGFR and AKT1 knockdown experiments. Knockdown of EGFR and AKT1 significantly inhibited proliferation of HelaCD73 and SiHaCD73 via changing cell cycle distribution and inducing cell apoptosis. The mechanism of increasing EGFR/AKT1 levels in CD73 overexpressed cells has not been clarified. Zhi’s data have suggested that CD73 may promote EGFR expression in breast cancer though regulating some associated transcription factor, such as PPARγ (32). Shali et al.’s study has suggested that CD73 regulates EGFR through c-Src in hepatocellular carcinoma, which is a critical molecular for EGFR Phosphorylation (30). However, more experimental verification is needed for the potential mechanism between CD73 and EGFR.
CD73 has both enzymatic and non-enzymatic function, not only increase the production of adenosine which leads to immune suppression, but also enhances angiogenesis, tumor growth and metastasis. Recent years, CD73 has been considered as a potential target for cancer therapy. CD73-blockage have showed an anti-tumor effect in animal experiments. Although it has not yet been used clinically, several clinical trials have tried to evaluate the therapeutic effect of CD73 targeting treatment in cancer (15,16). Notably, to overcome cancer complexity, combination of anti-CD73 and -other targets, such as CTLA-4, OX40, PD-L1, etc., would possibility enhance the efficacy of anti-cancer therapy (32,33). Multiple studies with combinatorial target strategies have been reported. Goswami and colleagues have found that the absence of CD73 improved survival in a murine model of glioblastoma multiforme treated with anti-CTLA-4 and anti-PD-1 (33). Sociali have showed that anti-CD73 mAb treatment improves the efficacy of anti-ErbB2 mAb for treating engrafted or spontaneous primary tumors and lung metastases, in mouse models of HER2/ErbB2-driven breast cancer (15). Allard B have found that anti-CD73 mAb significantly enhanced the activity of both anti-CTLA-4 and anti-PD-1 mAbs against MC38-OVA (colon) and RM-1 (prostate) subcutaneous tumors, and established metastatic 4T1.2 breast cancer (14). These studies have presented the potential application value of combined with CD73-blockage in cancer therapy. Although it hasn’t been reported yet, our results in this study suggested the combined of anti-CD73 and anti-EGFR/AKT1 treatment might be a worthwhile research direction.
Notably, it has been well known that CD73-generated adenosine plays an important role in tumor immunoescape. To mediate its immunosuppressive effect, CD73 generated adenosine can bind to G-protein-coupled receptors (i.e., A1R, A2AR, A2BR, A3R) that expressed on cell membrane of immune cells. High concentration of adenosine could suppress immune response via multiple mechanism, such as promoting regulated T cells proliferation, inhibition of M1 macrophage polarization, and blunt the capacity of nature killer (NK) cells (21). Thus, CD73 blockage may also kill tumors by activating immune response in vivo.
In this study, we found that EGFR/AKT1 knockdown lead to the upregulation of CDKN1A, and CDK2, and also result to the reduction in G1 phase and increased in G2 phase. Notably, CDK2 was usually considered as a driver of G1 to S phase. Several studies have shown that G2/M arrest was associated with upregulation of CDKN1A (34,35). In this study, the degree of elevated expression of CDKN1A in EGFR/AKT1 knockdown cells was significantly higher than that of CDK2. Thus, we speculated that increased CDK2 trigger G1 to S phase, simultaneous S to G2 phase were triggered with unknown mechanism and upregulation of CDKN1A resulted to increased in G2 phase. Finally, cell cycle distribution was reduced in G1 phase and increased in G2 phase.
There were following limitation of this study. First, although we have try to construct the murine xenograft models by subcutaneous injection of SiHaCD73 or SiHaNC cells, while there were no tumor tissue formed, Second, we have found that, EGFR/AKT1 play an important role during the promoted effect of CD73 in cervical cancer growth. However, the mechanism of increasing EGFR/AKT1 levels in CD73 overexpressed cells has not been clarified. The regulated mechanism of CD73 on EGFR/AKT1 pathway should be investigated in future studies.
Conclusions
In conclusion, our data demonstrated that CD73 overexpression promote cervical cancer cell progression in vitro and in vivo, which was related to increasing of EGFR and AKT1. EGFR and AKT1 knockdown could decrease the CD73 overexpressed cell’s proliferation via inducing cell cycle arrest and apoptosis.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2446/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2446/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2446/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2446/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 20200505) granted by Air Force Medical University’s Institutional Animal Care and Use Committee, in compliance with Air Force Medical University’s guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 2010;29:5346-58. [Crossref] [PubMed]
- Bavaresco L, Bernardi A, Braganhol E, et al. The role of ecto-5'-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem 2008;319:61-8. [Crossref] [PubMed]
- Gao ZW, Wang HP, Lin F, et al. CD73 promotes proliferation and migration of human cervical cancer cells independent of its enzyme activity. BMC Cancer 2017;17:135. [Crossref] [PubMed]
- Yu J, Wang X, Lu Q, et al. Extracellular 5'-nucleotidase (CD73) promotes human breast cancer cells growth through AKT/GSK-3β/β-catenin/cyclinD1 signaling pathway. Int J Cancer 2018;142:959-67. [Crossref] [PubMed]
- Zhi X, Wang Y, Zhou X, et al. RNAi-mediated CD73 suppression induces apoptosis and cell-cycle arrest in human breast cancer cells. Cancer Sci 2010;101:2561-9. [Crossref] [PubMed]
- Zhou X, Zhi X, Zhou P, et al. Effects of ecto-5'-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol Rep 2007;17:1341-6. [Crossref] [PubMed]
- Buisseret L, Pommey S, Allard B, et al. Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann Oncol 2018;29:1056-62. [Crossref] [PubMed]
- Leclerc BG, Charlebois R, Chouinard G, et al. CD73 Expression Is an Independent Prognostic Factor in Prostate Cancer. Clin Cancer Res 2016;22:158-66. [Crossref] [PubMed]
- Liu N, Fang XD, Vadis Q. CD73 as a novel prognostic biomarker for human colorectal cancer. J Surg Oncol 2012;106:918-9; author reply 920. [Crossref] [PubMed]
- Loi S, Pommey S, Haibe-Kains B, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013;110:11091-6. [Crossref] [PubMed]
- Ma XL, Shen MN, Hu B, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol 2019;12:37. [Crossref] [PubMed]
- Wu XR, He XS, Chen YF, et al. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol 2012;106:130-7. [Crossref] [PubMed]
- Yang Q, Du J, Zu L. Overexpression of CD73 in prostate cancer is associated with lymph node metastasis. Pathol Oncol Res 2013;19:811-4. [Crossref] [PubMed]
- Allard B, Pommey S, Smyth MJ, et al. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res 2013;19:5626-35. [Crossref] [PubMed]
- Chen S, Wainwright DA, Wu JD, et al. CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy 2019;11:983-97. [Crossref] [PubMed]
- Ghalamfarsa G, Kazemi MH, Raoofi Mohseni S, et al. CD73 as a potential opportunity for cancer immunotherapy. Expert Opin Ther Targets 2019;23:127-42. [Crossref] [PubMed]
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 2008;1783:673-94. [Crossref] [PubMed]
- Sadej R, Skladanowski AC. Dual, enzymatic and non-enzymatic, function of ecto-5'-nucleotidase (eN, CD73) in migration and invasion of A375 melanoma cells. Acta Biochim Pol 2012;59:647-52. [Crossref] [PubMed]
- Allard D, Chrobak P, Allard B, et al. Targeting the CD73-adenosine axis in immuno-oncology. Immunol Lett 2019;205:31-9. [Crossref] [PubMed]
- Wang L, Tang S, Wang Y, et al. Ecto-5'-nucleotidase (CD73) promotes tumor angiogenesis. Clin Exp Metastasis 2013;30:671-80. [Crossref] [PubMed]
- Gao ZW, Dong K, Zhang HZ. The roles of CD73 in cancer. Biomed Res Int 2014;2014:460654. [Crossref] [PubMed]
- Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311-9. [Crossref] [PubMed]
- Rivera F, García-Castaño A, Vega N, et al. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther 2009;9:1421-8. [Crossref] [PubMed]
- Johnson JR, Cohen M, Sridhara R, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res 2005;11:6414-21. [Crossref] [PubMed]
- Menges CW, Baglia LA, Lapoint R, et al. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res 2006;66:5555-9. [Crossref] [PubMed]
- Zhang B, Srirangam A, Potter DA, et al. HPV16 E5 protein disrupts the c-Cbl-EGFR interaction and EGFR ubiquitination in human foreskin keratinocytes. Oncogene 2005;24:2585-8. [Crossref] [PubMed]
- Morgan EL, Scarth JA, Patterson MR, et al. E6-mediated activation of JNK drives EGFR signalling to promote proliferation and viral oncoprotein expression in cervical cancer. Cell Death Differ 2021;28:1669-87. [Crossref] [PubMed]
- Wasson CW, Morgan EL, Müller M, et al. Human papillomavirus type 18 E5 oncogene supports cell cycle progression and impairs epithelial differentiation by modulating growth factor receptor signalling during the virus life cycle. Oncotarget 2017;8:103581-600. [Crossref] [PubMed]
- Spangle JM, Munger K. The HPV16 E6 oncoprotein causes prolonged receptor protein tyrosine kinase signaling and enhances internalization of phosphorylated receptor species. PLoS Pathog 2013;9:e1003237. [Crossref] [PubMed]
- Shali S, Yu J, Zhang X, et al. Ecto-5'-nucleotidase (CD73) is a potential target of hepatocellular carcinoma. J Cell Physiol 2019;234:10248-59. [Crossref] [PubMed]
- Wu R, Chen Y, Li F, et al. Effects of CD73 on human colorectal cancer cell growth in vivo and in vitro. Oncol Rep 2016;35:1750-6. [Crossref] [PubMed]
- Zhi X, Wang Y, Yu J, et al. Potential prognostic biomarker CD73 regulates epidermal growth factor receptor expression in human breast cancer. IUBMB Life 2012;64:911-20. [Crossref] [PubMed]
- Goswami S, Walle T, Cornish AE, et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med 2020;26:39-46. [Crossref] [PubMed]
- Yuan J, Zhang G, Li X, et al. Knocking down USP39 Inhibits the Growth and Metastasis of Non-Small-Cell Lung Cancer Cells through Activating the p53 Pathway. Int J Mol Sci 2020;21:8949. [Crossref] [PubMed]
- Hegazy MF, Dawood M, Mahmoud N, et al. 2α-Hydroxyalantolactone from Pulicaria undulata: activity against multidrug-resistant tumor cells and modes of action. Phytomedicine 2021;81:153409. [Crossref] [PubMed]


