
The efficacy and safety of concurrent chemoradiotherapy with induction chemotherapy vs. concurrent chemoradiotherapy alone for locally advanced nasopharyngeal carcinoma: a systematic-review and meta-analysis
Introduction
Locoregional nasopharyngeal carcinoma (LNC) is a primary malignant tumor of the mucosal epithelium in the nasopharynx, mostly occurring in the parietal and lateral walls of the nasopharynx, especially in the pharyngeal recess site. Its lesion types can be divided into nodular, ulcerative, and submucosal invasive types, with squamous cell carcinoma as the main pathological type and adenocarcinoma representing only a very small number of cases (1,2). Nasopharyngeal carcinoma tends to occur in the yellow race; statistics (3) show that 80% of nasopharyngeal carcinomas occurs in China. Nasopharyngeal carcinoma (NPC) is very sensitive to radiotherapy, and early NPC can be treated with radiotherapy alone. However, advanced NPC usually requires a comprehensive treatment mode of concurrent radiotherapy and chemotherapy to take effect.
Concurrent chemoradiotherapy (CCRT) can significantly improve the survival rate of patients and has become the standard treatment mode for advanced NPC (4). Although, for some patients with large tumors at presentation, CCRT cannot completely eliminate the tumor and may be harmful to the surrounding tissues, so 2–3 cycles of induction chemotherapy (IC) may be performed first followed by CCRT. IC, or neoadjuvant chemotherapy, has the advantages of no radiotherapy-induced fibrosis, which is conducive to the distribution and utilization of chemotherapeutic drugs in the primary nasopharyngeal carcinoma tumor. This increases the sensitivity and tolerance of patients to chemotherapy, and reduces the local and regional tumor burden, thereby improving the local control rate (5).
A previous case-control study by Li et al. (6) has reported that the addition of IC prior to CCRT can reduce the rate of distant metastasis and improve the survival rate of patients with nasopharyngeal carcinoma. However, another study by Zhang et al. (7) has suggested that for specific patients (NPC with chronic hepatitis B infection), IC does not provide a significant efficacy gain for CCRT, but increases the toxicity and liver burden. A meta-analysis is the best way to resolve the controversies (8). Therefore, we conducted this meta-analysis to further explore the efficacy and safety of IC + CCRT vs. CCRT alone, and we also tried to perform subgroup analysis of different stages of LNC, different age levels, and different IC chemotherapy regimens.
We present the following article in accordance with the PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-604/rc).
Methods
Inclusion of studies
The included studies were limited according to the five aspects of PICOS (participants, interventions, comparisons, outcomes, and study design). (I) Study design: we included randomized controlled trials (RCTs) and observational cohort studies. Case series, case reports, systematic reviews, experience summaries, and expert opinions and consensus were not included in the study. (II) Participants: all of the study subjects were patients with locally advanced nasopharyngeal carcinoma (stage II–IV), without distant metastasis. We did not limit the age or disease stage of the patients, and the subgroup analysis was performed according to the stage of disease, also the age of the patients. (III) Interventions and comparisons: there were at least two cohorts of IC + CCRT and CCRT alone in the included studies (for RCT studies, it was called two groups). Since the IC regimens varied between different studies, we recorded the specific regimen of IC, chemotherapy cycles (circles), time intervals for further analysis. Furthermore, we did not limit the regimen of chemoradiotherapy in CCRT. Studies of combination therapy with other drugs while taking IC, such as the addition of combination therapy with nimotuzumab to IC, were excluded. (IV) Outcomes: we used complete response rate, progression-free survival (PFS), distant metastasis-free survival (DMFS), and overall survival (OS) as the main efficacy indicators, and the incidence of Grade 3 or 4 adverse events or above was the secondary indicator.
Literature search strategy
The PubMed, Web of Science, Wiley online library, Elsevier databases, and Chinese National Knowledge Infrastructure (CNKI) were searched by computer to include the latest clinical research. We also searched for literature on this topic on Clinicaltrials.gov. The search method was a keyword rapid search, and the input keywords were as follows: “neoadjuvant chemotherapy/induction chemotherapy” and “concurrent chemoradiotherapy” and “nasopharyngeal carcinoma”. We did not limit the period of literature publication, and searched the above databases from the date of establishment of the database to January, 2022.
Literature selection and data extraction
After obtaining the full texts of the articles, two researchers independently completed the inclusion and screening of the studies. Any inconsistencies in this process were resolved through agreement by negotiation with a third researcher. Excel 2020 (released by Microsoft Corp). was used to extract the data, which included the following contents: (I) basic study data: publication time, author, and region; (II) characteristics of the study subjects: age, gender, disease stage, smoking history, T stage, and N stage; (III) study intervention methods: IC cycle, concurrent cycle, drug type, drug dose, and radiotherapy regimen and dose; and (IV) outcome data: Complete response rate, PFS, DMFS, OS, and adverse events. The PFS, DMFS, OS should be manifested by hazard ratio (HR), we pooled the HR of studies to perform the meta-analysis. In the process of data extraction, if no specific data was provided in the literature, the data were obtained according to the address specified in the literature. If the data could not be obtained, the original author of the study was contacted, and if the data still could not be obtained, the literature was excluded. We did not consider it possible to derive data from the graphical (Kaplan-Meier) presentation of data trends, unless otherwise indicated on the graphical presentation.
Risk of bias
We used the Cochrane risk of bias 2.0 scale (9) to assess the quality of RCTs and cohort studies, with an overall bias assessment of “low risk of bias”, “some concerns”, and “high risk of bias”. We consider the study with “high risk of bias” as with low quality and excluded it.
Statistical methods
I2 test analysis and Q test were used to assess the heterogeneity between different studies. I2<50% or P≥0.1 indicated that the heterogeneity was not statistically significant, signifying that there was no (or acceptable) heterogeneity between the literatures. The outcome indicators of complete response rate and adverse events rate in this study were pooled with risk ratio (RR) effect size, and PFS, DMFS, OS with the HR effect size, all with 95% confidence interval (CI) calculated, and P<0.05 (both sided) indicating the difference statistically significant. If there was no statistical heterogeneity in the included studies, the fixed-effect model was used, and if there was heterogeneity, the random-effects model was used. In this study, STATA 16.0 software (released by StataCorp LLC, TX77845, USA) was used as the analysis tool to present the analysis results in the form of a forest plot. We eliminated the studies one-by-one to perform the heterogeneity investigation and sensitivity analysis. Subgroup analysis was introduced to investigate the heterogeneity also. The publication bias analysis was conducted by Egger’s test.
Results
Literature screening results
This study initially retrieved 797 articles, and 13 studies were finally included (6-7,10-20). The selection flow chart is shown in Figure 1. Some studies (21,22) were single-arm studies, which could not provide comparison between the two groups of data, and therefore excluded. One study (23) was a systematic review, which could not provide data, and was excluded. Moreover, some studies (24,25) provided a comparison of efficacy using different chemotherapeutic drug regimens in IC + CCRT, but could not provide a comparison of the efficacy between IC + CCRT and CCRT alone, and were also excluded. We did not list all of the excluded articles, but only listed five representative studies.
Basic characteristics of literatures
As shown in Table 1, a total of 7,197 patients were included in this study, including 3,764 patients who received IC + CCRT and 3,433 patients who received CCRT alone. The TNM grade was II–IV, and the average follow-up time was 15–76.8 months. The included 2 RCTs all with low risk of bias, and 11 cohort studies with some concerns of risk.
Table 1
| Author | Year of publication | Type of study | TNM stage | Age (years) | Population (E/C) | Induction chemotherapy regimen | Median follow-up time (months) | Outcome indicators | RoB 2.0 |
|---|---|---|---|---|---|---|---|---|---|
| Li et al. (6) | 2018 | Retro | Stage III–IV | 17.28 [8–21] | 43:43 | TPF | 51.5 | b, c, d, e | Some concerns of risk |
| Zhang et al. (7) | 2017 | Retro | Stage III–IV | 44 [39–55] | 70:70 | TPF or PF or TP | 61 | b, c, d | Some concerns of risk |
| Sun et al. (10) | 2016 | RCT | Stage III–IV | 44 [39–50] | 241:239 | TPF | 45 | a, b, c, d | Low risk of bias |
| Ou et al. (11) | 2016 | Retro | Stage III–IV | 49 [18–75] | 58:48 | TPF | 76.8 | a, b, c, d | Some concerns of risk |
| Lan et al. (12) | 2016 | Retro | Stage IV | 46 [13–78] | 406:196 | TPF | 52.3 | c, d | Some concerns of risk |
| Kawahira et al. (13) | 2017 | Retro | Stage III–IV | 57 [18–92] | 12:16 | TPF | 36.4 | a, e | Some concerns of risk |
| Hong et al. (14) | 2018 | RCT | Stage IV | 53.1 [30–80] | 239:240 | TPF | 72.0 | a, b, c, d, e | Low risk of bias |
| Jin et al. (15) | 2020 | Retro | Stage II | 54.3 [33–67] | 195:250 | PF or TP or TPF | 15 | b, d, e | Some concerns of risk |
| Zhang et al. (16) | 2017 | Retro | Stage III–IV | 47.30±10.42 | 178:84 | PF or TP or TPF | 29.02 | a, b, c, d | Some concerns of risk |
| Li et al. (17) | 2018 | Retro | Stage II | 48.6 | 78:95 | TPF | 64.7 | b, c, d, e | Some concerns of risk |
| Xia et al. (18) | 2019 | Retro | Stage III | 48 [37–62] | 1,416:986 | TPF | 67 | b, d | Some concerns of risk |
| Xia et al. (18) | 2019 | Retro | Stage IV | 48 [35–60] | 676:902 | TPF or PF or TP | 67 | b, d | Some concerns of risk |
| Wang et al. (19) | 2017 | Retro | Stage III–IV | 67.6±5.3 | 82:82 | TP | 52.5 | e | Some concerns of risk |
| Wang et al. (20) | 2020 | Retro | Stage III–IV | 35–58 | 38:92 | TPF | 59 | b, c, d, e | Some concerns of risk |
Outcome indicators: a, complete response; b, progression-free survival; c, distant metastasis-free survival; d, overall survival; e, any grade 3 or 4 adverse events. TNM, tumor node and metastases; E/C, experimental group/control group; RCT, randomized controlled trial; TPF, docetaxel, cisplatin, and fluorouracil chemotherapy; PF, cisplatin and fluorouracil; TP, docetaxel and cisplatin; Retro, retrospective study, RoB, risk of bias.
Meta-analysis results
Complete response rate
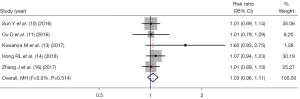
Five studies (10-11,13-14,16) reported the complete response rate of IC + CCRT and CCRT alone, and there was no statistical heterogeneity between the articles (I2=0%, P=0.514), and thus, fixed-effect mode analysis was used. As shown in Figure 2, there was no significant difference in the complete response rate between IC + CCRT and CCRT alone in NPC patients (RR =1.03, 95% CI: 0.96–1.11, P=0.336).
PFS
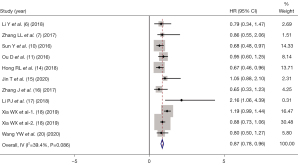
PFS indicators were reported in 10 of the included studies (6-7,10-11,14-18,20), and there was statistical heterogeneity between the articles (I2=39.4%, P=0.086). Therefore, by using fixed-effect mode, the pooled HR of IC as a prognostic factor predicting PFS for NPC patients was (HR =0.87, 95% CI: 0.78–0.96, P=0.214), as shown in Figure 3.
DMFS
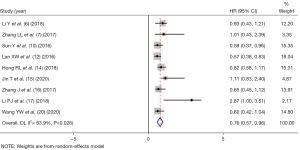
DMFS indicators were reported in 9 studies (6-7,10,12,14-17,20). There was statistical heterogeneity between the articles (I2=53.9%, P=0.026). As shown in Figure 4, by using random-effect mode, the pooled HR showed that IC was an independent prognostic factor predicting better for NPC patients (HR =0.76, 95% CI: 0.57–0.96, P=0.004).
OS
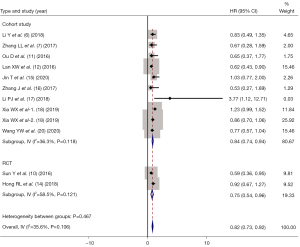
OS indicators were reported in 12 of the included studies (6-7,10-12,14-20), and there was statistical heterogeneity between the articles (I2=35.6%, P=0.106). Therefore, using fixed-effect mode combined analysis the pooled HR of OS was HR =0.82 (95% CI: 0.73–0.92, P=0.036).
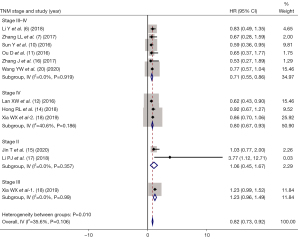
The studies were divided into stage II, stage III, stage IV, and stage III–IV according to TNM stage. As shown in Figure 5, the HR of OS between IC + CCRT and CCRT alone were as follows: stage II (HR =1.06, 95% CI: 0.45–1.67, P= 0.375); stage III (HR =1.23, 95% CI: 0.96–1.49, P=0.068); stage IV (HR =0.80, 95% CI: 0.67–0.93, P=0.018); and stage III–IV (HR =0.71, 95% CI: 0.55–0.86, P=0.002).
According to the study type, the included studies were divided into two subgroups: RCTs and cohort studies. As shown in Figure 6, the HR of IC for different study types were HR =0.75 (95% CI: 0.54–0.96, P=0.114) for RCTs, while HR =0.84 (95% CI: 0.74–0.94, P=0.110) for cohort studies.
Serious adverse reactions
A total of six studies (6,13-15,17,19) reported any grade 3 or 4 adverse events, and there was statistical heterogeneity between the articles (I2=64.8%, P=0.014). Therefore, using random-effect mode combined analysis, we found that there was a statistically significant difference in the incidence rate of serious adverse reactions between IC + CCRT and CCRT alone in NPC patients (RR =1.22, 95% CI: 1.00–1.47, P=0.045), as shown in Figure 7.

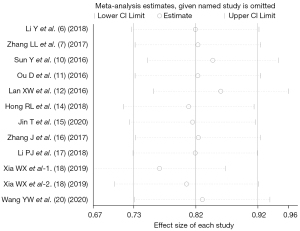
Heterogeneity investigation and sensitivity analysis
In the analysis of OS outcome indicators, no statistically significant heterogeneity was found between the included studies. We introduced subgroups by study type to investigate if risk of bias played a role in the analysis of effect sizes. However, the result showed there was no difference between the two subgroups, which suggested the risk of bias played little effect on the results. The sensitivity analysis also showed no sign of impact on the effect sizes by eliminating studies one by one, as shown in Figure 8.
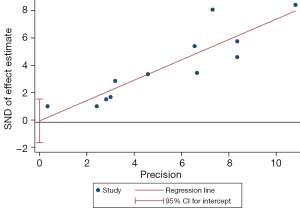
Analysis of publication bias
In the analysis of OS indicators, the Egger’s test showed that both sides were evenly distributed, and P=0.891, suggesting that there was no publication bias, as shown in Figure 9.
Discussion
Although NPC is highly sensitive to radiotherapy, 70% of NPC patients are at an advanced stage at the time of diagnosis, with strong tumor invasiveness and risk of metastasis to the hematologic and lymphatic systems. Only CCRT can inhibit the primary lesion, control distant metastasis of the tumor, and improve the efficacy of treatment (26). IC refers to chemotherapy used before radiotherapy, and its role is to inhibit the implantation of tumor cells, thereby killing these tumor cells in the systemic circulation and reducing subclinical metastases. The use of chemotherapy in untreated patients has good compliance and can accomplish the treatment as planned, enhance subsequent radiotherapy sensitivity, and reduce the toxicity caused by radiotherapy in some patients, such as limited mouth opening caused by cervical fibrosis and mandibular joint fibrosis (27).
In this meta-analysis, 13 clinical studies (including two RCTs and 11 retrospective cohort studies) involving a total of 7,197 patients were included. According to the overall pooled results, there were no significant differences between the two groups for the outcome of complete response rate, the pooled HR for IC as a predictor for better PFS showed no significant too, but the pooled HRs for IC as a predictor for DMFS and OS were statistically significant, which meant that IC could be a prognostic factor predicting better DMFS and OS. After subgrouping the patients according to TNM stage II, stage III, and stage IV, the P value of HR for OS showed no significant for patients with stage II, which indicated that IC + CCRT treatment could be more suitable for patients with stage III or IV. A study by He et al. (28) has reported that for stage II nasopharyngeal carcinoma, intensity modulated radiation therapy (IMRT) alone can achieve better results, and that IC or CCRT do not provide additional benefits.
Since no fixed induction chemotherapy regimen was applied for most of the studies, so we could not perform subgroup analysis according to the chemotherapy regimen. Zeng et al. (29) compared the two regimens of TPF (cisplatin, fluorouracil, and Docetaxel) with GP (gemcitabine and cisplatin) for IC, and it was found that there was no significant difference in the improvement of patient survival between these two regimens during IC. A previous mesh meta-analysis (30) revealed that the docetaxel-platinum-5-fluorouracil (5FU) regimen had better efficacy than other regimens. Choi et al.’s reticular meta-analysis (31) included nine controlled studies with a total of eight induction chemotherapy regimens, and concluded that the docetaxel + cisplatin (DC), gemcitabine + cisplatin (GP), and cisplatin + capecitabine (PX) regimens were the most effective for improving patient survival.
The side effects of radiotherapy manifest as radiation mucosal injury and radiation dermatitis, and chemotherapeutic drugs cause significant gastrointestinal symptoms and hematological toxicity. When the two are synchronized, the side effects are superimposed and may be enhanced by each other, such as significant mucosal reactions, resulting in decreased patient tolerance (32). The present meta-analysis showed that IC + CCRT had more serious adverse reactions than CCRT alone, which indicated that IC increased the toxicity in concurrent chemotherapy, especially that in the oral cavity, digestive tract, and bone marrow. This resulted in some patients having to reduce the chemotherapeutic drug dosage or use segmented radiotherapy, and even some having to suspend chemoradiotherapy due to severe toxicity.
In this study, although no heterogeneity among the included articles was observed in the analysis of PFS, and OS, it should be noted that there were some variations between different studies, which may affect the accuracy of our results. For example, the chemotherapy and radiotherapy regimens differed among the included studies, with some studies using the three-dimensional conformal or intensity-modulated radiotherapy, and others utilized the traditional two-dimensional conformal radiotherapy. Moreover, although docetaxel, cisplatin, and fluorouracil (TPF) was used in the majority of included studies, other regimens were also applied which could be one source of the heterogeneity. Lastly, there were only two RCTs included in this study, the cohort studies included were non-RCTs with lower quality. Therefore, more RCTs with better quality are still needed to compare the efficacy and safety of IC + CCRT and CCRT alone.
Conclusions
Before CCRT for locally advanced nasopharyngeal carcinoma, the use of IC shows a positive effect for patients with TNM stage III and IV, but there is no significant gain for patients with stage II. In addition, when IC is applied, it will significantly increase the toxicity of the oral cavity, digestive tract, and bone marrow, and thus, it should be considered whether patients can tolerate it.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-604/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-604/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee AWM, Ng WT, Chan JYW, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev 2019;79:101890. [Crossref] [PubMed]
- Lam WKJ, Chan JYK. Recent advances in the management of nasopharyngeal carcinoma. F1000Res 2018;7:eF1000 Faculty Rev-1829.
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. [Crossref] [PubMed]
- Perri F, Della Vittoria Scarpati G, Caponigro F, et al. Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther 2019;12:1583-91. [Crossref] [PubMed]
- Zheng J, Wang G, Yang GY, et al. Induction chemotherapy with nedaplatin with 5-FU followed by intensity-modulated radiotherapy concurrent with chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Jpn J Clin Oncol 2010;40:425-31. [Crossref] [PubMed]
- Li Y, Tang LQ, Liu LT, et al. Induction Chemotherapy Plus Concurrent Chemoradiotherapy Versus Concurrent Chemoradiotherapy Alone in Locoregionally Advanced Nasopharyngeal Carcinoma in Children and Adolescents: A Matched Cohort Analysis. Cancer Res Treat 2018;50:1304-15. [Crossref] [PubMed]
- Zhang LL, Zhou GQ, Li YC, et al. Induction Chemotherapy Has No Prognostic Value in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma and Chronic Hepatitis B Infection in the IMRT Era. Transl Oncol 2017;10:800-5. [Crossref] [PubMed]
- Xu G, Wang Q, Wu X, et al. Comparison of Induction Chemotherapy Plus Concurrent Chemoradiotherapy and Concurrent Chemoradiotherapy Alone in Locally Advanced Nasopharyngeal Carcinoma. Technol Cancer Res Treat 2021;20:1533033821990017. [Crossref] [PubMed]
- Minozzi S, Dwan K, Borrelli F, et al. Reliability of the revised Cochrane risk-of-bias tool for randomised trials (RoB2) improved with the use of implementation instruction. J Clin Epidemiol 2022;141:99-105. [Crossref] [PubMed]
- Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509-20. [Crossref] [PubMed]
- Ou D, Blanchard P, El Khoury C, et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol 2016;62:114-21. [Crossref] [PubMed]
- Lan XW, Zou XB, Xiao Y, et al. Retrospective Analysis of the Survival Benefit of Induction Chemotherapy in Stage IVa-b Nasopharyngeal Carcinoma. PLoS One 2016;11:e0160758. [Crossref] [PubMed]
- Kawahira M, Yokota T, Hamauchi S, et al. Survival benefit of adding docetaxel, cisplatin, and 5-fluorouracil induction chemotherapy to concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma with nodal Stage N2-3. Jpn J Clin Oncol 2017;47:705-12. [Crossref] [PubMed]
- Hong RL, Hsiao CF, Ting LL, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol 2018;29:1972-9. [Crossref] [PubMed]
- Jin T, Zhang Q, Luo DH, et al. Concurrent Chemoradiotherapy With or Without Induction Chemotherapy for Patients with Stage II Nasopharyngeal Carcinoma: An Update. Transl Oncol 2020;13:25-31. [Crossref] [PubMed]
- Zhang J, Chen S, Li G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy vs. concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a retrospective cohort study. Cancer Chemother Pharmacol 2017;79:1087-97. [Crossref] [PubMed]
- Li PJ, Mo HY, Luo DH, et al. The efficacy of induction chemotherapy in the treatment of stage II nasopharyngeal carcinoma in intensity modulated radiotherapy era. Oral Oncol 2018;85:95-100. [Crossref] [PubMed]
- Xia WX, Liang H, Lv X, et al. Stage-specific concurrent chemoradiotherapy with or without induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma: a retrospective, population-based study. Cancer Manag Res 2019;11:9813-27. [Crossref] [PubMed]
- Wang C, Tang X, Wang J, et al. Induction Chemotherapy plus Concurrent Chemoradiotherapy vs Concurrent Chemoradiotherapy in Elderly Patients with Advanced Nasopharyngeal Carcinoma. Otolaryngol Head Neck Surg 2017;157:233-8. [Crossref] [PubMed]
- Wang YW, Ho SY, Lee SW, et al. Induction Chemotherapy Improved Long Term Outcomes in Stage IV Locoregional Advanced Nasopharyngeal Carcinoma. Int J Med Sci 2020;17:568-76. [Crossref] [PubMed]
- Ferrari D, Chiesa F, Codecà C, et al. Locoregionally advanced nasopharyngeal carcinoma: induction chemotherapy with cisplatin and 5-fluorouracil followed by radiotherapy and concurrent cisplatin: a phase II study. Oncology 2008;74:158-66. [Crossref] [PubMed]
- Toumi N, Ben Kridis W, Mnejja W, et al. TPF induction chemotherapy followed by concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma: Long term results of a Tunisian series. Cancer Radiother 2018;22:216-21. [Crossref] [PubMed]
- Mané M, Benkhaled S, Dragan T, et al. Meta-Analysis on Induction Chemotherapy in Locally Advanced Nasopharyngeal Carcinoma. Oncologist 2021;26:e130-41. [Crossref] [PubMed]
- Zang J, Xu M, Li C, et al. Gemcitabine and cisplatin versus docetaxel and cisplatin as induction chemotherapy followed by concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma from non-endemic area of China. J Cancer Res Clin Oncol 2020;146:2369-78. [Crossref] [PubMed]
- Wu Q, Liao W, Huang J, et al. Cost-effectiveness analysis of gemcitabine plus cisplatin versus docetaxel, cisplatin and fluorouracil for induction chemotherapy of locoregionally advanced nasopharyngeal carcinoma. Oral Oncol 2020;103:104588. [Crossref] [PubMed]
- Peng H, Dong D, Fang MJ, et al. Prognostic Value of Deep Learning PET/CT-Based Radiomics: Potential Role for Future Individual Induction Chemotherapy in Advanced Nasopharyngeal Carcinoma. Clin Cancer Res 2019;25:4271-9. [Crossref] [PubMed]
- Zhao L, Gong J, Xi Y, et al. MRI-based radiomics nomogram may predict the response to induction chemotherapy and survival in locally advanced nasopharyngeal carcinoma. Eur Radiol 2020;30:537-46. [Crossref] [PubMed]
- He Y, Zhao Z, Wang Y, et al. Induction chemotherapy followed by intensity-modulated radiotherapy versus concurrent chemoradiotherapy in nasopharyngeal carcinoma: A retrospective analysis. Clin Otolaryngol 2021;46:976-82. [Crossref] [PubMed]
- Zeng Z, Yan RN, Tu L, et al. Assessment of Concurrent Chemoradiotherapy plus Induction Chemotherapy in Advanced Nasopharyngeal Carcinoma: Cisplatin, Fluorouracil, and Docetaxel versus Gemcitabine and Cisplatin. Sci Rep 2018;8:15581. [Crossref] [PubMed]
- Bongiovanni A, Vagheggini A, Fausti V, et al. Induction chemotherapy plus concomitant chemoradiotherapy in nasopharyngeal carcinoma: An updated network meta-analysis. Crit Rev Oncol Hematol 2021;160:103244. [Crossref] [PubMed]
- Choi HC, Chan SK, Lam KO, et al. The Most Efficacious Induction Chemotherapy Regimen for Locoregionally Advanced Nasopharyngeal Carcinoma: A Network Meta-Analysis. Front Oncol 2021;11:626145. [Crossref] [PubMed]
- Zhan ZJ, Tao HY, Qiu WZ, et al. Clinical value of nedaplatin-based chemotherapy combined with radiotherapy for locoregional advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis. J Cancer 2020;11:6782-9. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



