
Safety and efficacy of laparoscopic hepatectomy versus open hepatectomy for giant hepatic cysts: a systematic review and meta-analysis
Introduction
Liver cyst belongs to the more common and benign liver tumor, usually because of the liver vagal bile duct or intrahepatic bile duct, lymphatic duct development disorder, leading to the retention of contents formed in the lumen (1). Hepatic cysts are common in people over 50 years of age, and the incidence is high in women. The detection rate of hepatic cysts in the normal population is 2.5–5.0% as an incidental finding on imaging, and only 15% of them are symptomatic (2,3). Asymptomatic cysts <5 cm in diameter do not require special treatment, but surgery is the usual therapeutic measure for larger cysts. When the diameter of the cyst is >10 cm, it can be called a giant hepatic cyst (4). Giant hepatic cysts can impinge on the surrounding organs, resulting in abdominal discomfort or fullness, and even affect digestive and respiratory function, causing jaundice etc. In recent years, with the development of minimally invasive surgery and the promotion and popularization of laparoscopic techniques, laparoscopic fenestration (LF) as the hepatic cyst surgical technique has become more widely accepted (5). Although traditional open fenestration (OF) can complete the operation under direct vision and has a definite effect, it has disadvantages such as longer recovery time and greater trauma compared with laparoscopic surgery (6). Obviously, one of the advantages of laparoscopic liver resection is the small surgical incision, that is, less trauma. Traditional laparotomy, if half the liver is cut, requires a 20–30 cm incision. (I) Laparoscope only needs five holes, basically no scars, patients’ postoperative pain is small, compared with the traditional open surgery, the use of laparoscopy can greatly reduce the pain. (II) Patients can get out of bed as soon as possible, the function of each organ system recovers quickly, less complications after surgery, which is conducive to the rapid recovery of patients. (III) More importantly, for professional doctors, laparoscopic has a high resolution, can make the blood vessels around the liver, structure is more clear, more conducive to surgery for professional doctors, laparoscopic has high resolution, can make the liver and liver vessels, bile duct structure show more clear, make the operation more accurate. (IV) The hospital stay was even shorter. Despite so many advantages of laparoscopic hepatectomy nowadays, there is no denying that there is no way to make this technique fully universal, which still has its limitations. (I) One of the very important point is the problem of vision exposure, like to remove the back of the liver or the top of the diaphragm, the laparoscopic surgical field is more difficult, surgical risk increased significantly, in addition to experienced, skilled doctors in the basis of adequate preoperative preparation can consider implementation, general inconvenience to choose laparoscopic surgery. (II) Laparoscopic hepatectomy also faces the absence of 3D stereo vision and the lack of touch, which may cause some difficulties in positioning. (III) The other is the problem of bleeding, with laparoscopic surgery, once the bleeding is difficult to control, the surgical vision is blurred, at this time it has to be reconverted to open surgery. (IV) In addition to the above factors, there are many details, such as: operation difficulty, high risk, lack of a set of surgical standards, long learning cycle and so on. As a result, this technology can only be carried out in some third-class A large hospitals, and there are many difficulties in promoting it, which need hospitals to introduce talents and technology. The aim of this study was to systematically evaluate the efficacy and safety of laparoscopic hepatectomy versus open hepatectomy in the treatment of giant hepatic cysts by meta-analysis, in order to provide more theoretical basis for clinical treatment.We present the following article in accordance with the PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-910/rc).
Methods
Inclusion and exclusion criteria for literature search
Inclusion criteria
(I) Patients with giant liver cysts were selected for the study subjects; (II) laparoscopic hepatic resection was used in the intervention group and open hepatic resection in the control group; (III) at least one indicator was included in the operative time, intraoperative blood loss, hospital stay, postoperative gastrointestinal function recovery time, postoperative incision fat liquefaction, and complications; (IV) randomized controlled trials (RCTs) and case-control studies.
Exclusion criteria
(I) Reviews, experience summaries and meta-analyses; (II) individual case or case study; (III) basic study conducted on rats, rabbits and other animals; (IV) duplicate publication or full text cannot be obtained; (V) unknown data description.
Description of intervention
Patients were divided into LF and OF groups for laparoscopic hepatectomy or open hepatectomy respectively.
Outcome indicators
Operation time, intraoperative blood loss, hospital stay, postoperative gastrointestinal function recovery time, and postoperative complications such as incision fat liquefaction or incisional infection were assigned as indicators of patient outcomes.
Search strategy
The studies related to the efficacy and safety of laparoscopic hepatectomy and open hepatectomy for giant hepatic cysts published in CNKI, Wanfang, VIP, CBM, PubMed, Embase, Cochrane Library and other databases were searched from database establishment to December 2021. The search terms included “hepatic cyst”, “cyst of liver”, “liver cysts”, “laparoscopic fenestration”, “LF”, “open fenestration” and “OF”. To expand the sample size to reduce the risk of offset, we included both RCTs and observational studies in this study. We also reviewed the grey literature to complete the search of all the literature whenever possible.
Literature screening and data extraction
After literature retrieval, two reviewers independently screened out duplicate publications using the function in Endnote X9 software. Next, the title and abstract were read as a preliminary screening, unqualified studies were excluded and the full text was downloaded for further review. Any disagreements in opinions were resolved through discussion with a third reviewer.
Extracted information included: author, publication year, sample size, sample size per group, sex, age, type and number of cysts, outcome indicators and intervention measure.
Risk of bias
Randomized controlled trials were assessed according to the Cochrane Collaboration’s risk of bias tool (7). Assessments considered the generation of random sequences, allocation concealment, blinding to participants and implementers, blinding to outcome evaluators, selective reporting, completeness of outcome data, and other biases. The literature quality evaluation was completed independently by two reviewers, and when disagreements occurred, it was resolved by discussion with a third reviewer.
The Newcastle-Ottawa quality assessment scale (NOS) was used for qualitative evaluation of the included case-control trials. The NOS scale uses a semiquantitative star system for the evaluation of literature quality, with a full score of 9 points. The evaluation included population selection, comparability and exposure evaluation. Again, two researchers independently evaluated the literature, with negotiation of any inconsistencies or adjudication by the third researcher adjudicated (8).
Statistical analysis
We performed a unified analysis of all study findings using Stata 15.1 software and forest plots. The odds ratio (OR) was used for dichotomous variables (postoperative complications). Continuous variables (operation time, intraoperative blood loss, hospital stay and postoperative gastrointestinal function recovery time) were analyzed by standardized mean difference (SMD), and literature heterogeneity was analyzed by the I2 test. I2>50% indicates a statistical difference in heterogeneity. If the test results showed statistical homogeneity (I2<50%), a fixed-effects model was used to combine and analyze the data. Each effect size was expressed by the 95% confidence interval (CI), and P<0.05 indicated statistical significance. Sensitivity analysis was performed to test the stabilization of the results, using the Influence Analysis tool provided by Stata 15.1, and funnel plots were used to test whether the results had publication bias.
Results
Literature search results
A total of 1,461 studies were retrieved, and 978 remained after duplicate removal. After preliminary screening and reading the full text, 43 studies were finally included. Figure 1 shows the literature screening process and results.
Basic characteristics of included literature
The 43 studies included 3,375 patients with hepatic cysts. Basic information of the studies is shown in Table 1.
Table 1
| Study | Year | Sample | M/F | Age (years) | NOS grade | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LF | OF | LF | OF | LF, mean ± SD or median | OF, mean ± SD or median | |||||
| Bian (9) | 2018 | 112 | 112 | 47/65 | 44/68 | 43.5±2.6 | 42.4±2.3 | 6 | ||
| Cao et al. (10) | 2016 | 40 | 40 | 23/17 | 22/18 | 51.0±4.20 | 50.90±5.10 | – | ||
| Chen (11) | 2013 | 27 | 28 | 14/13 | 14/14 | 46.80±4.70 | 46.80±4.70 | 6 | ||
| Chen (12) | 2015 | 25 | 25 | 11/14 | 10/15 | 42.48±1.41 | 42.71±1.32 | – | ||
| Chen et al. (13) | 2011 | 50 | 50 | 23/27 | – | 41.30±2.50 | – | 5 | ||
| Chen et al. (14) | 2006 | 17 | 19 | 10/7 | 13/6 | 41.10 | 44.20 | 8 | ||
| Ding et al. (15) | 2013 | 28 | 23 | 12/16 | 9/14 | 45.70±3.60 | 49.50±4.70 | 5 | ||
| Ferrizado (16) | 2009 | 37 | 34 | 17/20 | 13/21 | 51.10±20.30 | 52.60±21.80 | 6 | ||
| Gall et al. (17) | 2009 | 48 | 11 | 18/30 | 5/6 | 60.00 | 60.00 | 8 | ||
| Gigot et al. (18) | 2001 | 15 | 9 | 2/13 | 4/5 | 57.00 | 45.00 | 7 | ||
| Guo et al. (19) | 2010 | 31 | 27 | 12/19 | 9/18 | 45.00±9.80 | 42.00±11.20 | 7 | ||
| Huang (20) | 2016 | 33 | 33 | 12/21 | 14/19 | 46.10±4.20 | 45.30±3.70 | – | ||
| Jian (21) | 2015 | 20 | 18 | 9/11 | 8/10 | 54.90±8.30 | 53.20±7.60 | – | ||
| Jiang et al. (22) | 2009 | 30 | 15 | 15/15 | 10/5 | 45.00 | 45.00 | 6 | ||
| Kirbiririti (23) | 2013 | 50 | 50 | 34/16 | 33/17 | – | – | – | ||
| Lao (24) | 2017 | 59 | 40 | 21/38 | 10/30 | 56.1±12.6 | 55.6±12.8 | 6 | ||
| Li et al. (25) | 2018 | 50 | 50 | 22/28 | 24/26 | 48.17±2.31 | 48.20±2.36 | 6 | ||
| Li (26) | 2021 | 90 | 90 | 32/58 | 30/60 | 47±9 | 47±10 | – | ||
| Lian et al. (27) | 2016 | 38 | 30 | 25/13 | 19/11 | 41.88±12.37 | 42.13±11.69 | 6 | ||
| Liu et al. (28) | 2020 | 50 | 50 | 34/16 | 35/15 | 45.83±2.25 | 45.94±2.39 | – | ||
| Liu et al. (1) | 2016 | 39 | 24 | 8/31 | 5/19 | 47.1±7.7 | 47.0±7.8 | 6 | ||
| Liu et al. (29) | 2012 | 19 | 13 | 10/9 | 4/9 | 55.30 | 56.80 | 7 | ||
| Liu (30) | 2019 | 46 | 43 | 26/20 | 22/21 | 47.69±4.76 | 48.08±4.58 | 6 | ||
| Qin et al. (31) | 2016 | 63 | 54 | 30/33 | 25/29 | 56.70±4.30 | 57.10±4.50 | 6 | ||
| Song et al. (32) | 2017 | 42 | 32 | 19/23 | 15/17 | 48.2±5.3 | 48.3±5.2 | 6 | ||
| Sun et al. (33) | 2014 | 28 | 21 | 8/20 | 9/12 | 69.70 | 71.30 | 6 | ||
| Wang (34) | 2016 | 42 | 41 | 22/20 | 21/20 | 56.35±4.45 | 56.35±4.45 | 6 | ||
| Wang et al. (35) | 2016 | 24 | 48 | 2/22 | 7/41 | 61.40±8.10 | 59.30±7.90 | 6 | ||
| Wu (36) | 2012 | 40 | 32 | 12/19 | 14/18 | 45.00±9.80 | 42.00±11.20 | 5 | ||
| Xiao et al. (37) | 2013 | 30 | 30 | 16/14 | 17/13 | 43.50±2.30 | 44.1±2.40 | – | ||
| Xiao et al. (38) | 2016 | 30 | 30 | 15/15 | 14/16 | 55.70±3.10 | 58.80±6.30 | 5 | ||
| Xu and Liu (39) | 2011 | 31 | 32 | 12/19 | 14/18 | 45.00±9.80 | 42.00±11.20 | 5 | ||
| Xu (40) | 2016 | 41 | 41 | 21/20 | 22/19 | 48.14±2.21 | 48.38±2.25 | – | ||
| Yan and Wu (41) | 2013 | 26 | 20 | 10/16 | 10/10 | 54.70 | 54.70 | 6 | ||
| Yang and Chen (42) | 2015 | 25 | 25 | 13/12 | 13/12 | 32.57±9.85 | 32.08±10.12 | 7 | ||
| Yi et al. (43) | 2007 | 52 | 117 | 21/32 | 43/74 | 43.00±9.20 | 45.00±12.80 | 6 | ||
| Zhang et al. (44) | 2010 | 52 | 42 | 20/32 | 18/24 | 58.13 | 63.29 | 7 | ||
| Zhao and Wang (45) | 2016 | 38 | 38 | 19/19 | 18/20 | 44.50±5.80 | 44.50±5.80 | – | ||
| Zhao (46) | 2016 | 40 | 40 | 21/19 | 20/20 | 45.70±3.10 | 47.40±2.40 | 7 | ||
| Zheng (47) | 2020 | 50 | 50 | 27/23 | 28/22 | 45.92±4.18 | 46.07±4.18 | 6 | ||
| Zheng and Zeng (48) | 2012 | 49 | 49 | 20/29 | 22/27 | 59.32±9.56 | 60.15±9.93 | – | ||
| Zhu and Wan (49) | 2019 | 50 | 50 | 10/40 | 10/40 | 42.86±3.63 | 42.52±3.78 | 6 | ||
| Zou et al. (50) | 2011 | 22 | 20 | 10/12 | 9/11 | 24.60±12.30 | 24.60±12.30 | – | ||
LF, laparoscopic fenestration; NOS, Newcastle-Ottawa quality assessment scale; OF, open fenestration.
Quality assessment
There were 12 randomized controlled studies. According to the risk of bias tool of the Cochrane Collaboration, all were low risk of bias studies. One study used a random number table. None stated whether allocation concealment was performed, did not state the subject blindness method, did not state if there was a blind method for the outcome evaluator, did not state if there was a selective report or if the outcome indicators were complete, and did not contain other risk of bias. Figures 2,3 show the risk of bias evaluation of the included studies. There were 31 case-control studies: 5 low-quality studies and 26 medium-high-quality studies.
Meta-analysis of operative time
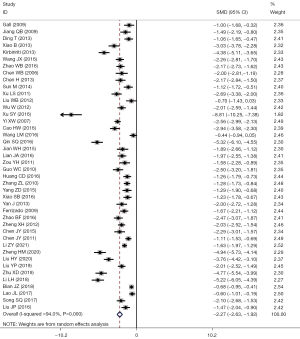
Of the 43 studies, 42 reported problems related to operation time: I2=94.0%, using the random-effects model to compare the two groups (SMD =–2.27, 95% CI: –2.63 to –1.92, P<0.001). The operation time in the LF group was significantly shorter than that in OF group, and the difference had statistical significance, as shown in Figure 4.
Meta-analysis of intraoperative blood loss
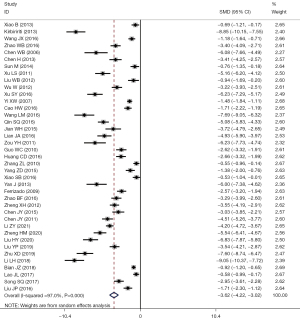
A total of 39 studies reported problems related to intraoperative blood loss: I2=97.0%, using the random-effects model for comparison between the two groups (SMD =–3.62, 95% CI: –4.22 to –3.02, P<0.001). The intraoperative blood loss in LF group was significantly less than that in the OF group, and the difference had statistical significance, as shown in Figure 5.
Meta-analysis of length of hospital stay
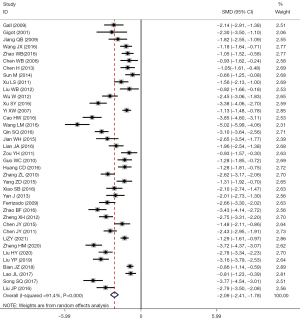
A total of 38 studies reported issues regarding hospitalization: I2=91.4%, using a random-effects model for comparison between the two groups (SMD =–2.09, 95% CI: –2.41 to –1.78, P<0.001). The length of hospital stay in the LF group was significantly shorter than that in the OF group, and the difference had statistical significance, as shown in Figure 6.
Meta-analysis of postoperative recovery time of gastrointestinal function
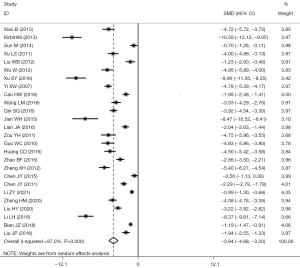
A total of 39 studies reported the problems related to the recovery time of postoperative gastrointestinal function: I2=97.0%, using the random-effects model for comparison between the two groups (SMD =–3.94, 95% CI: –4.68 to –3.20, P<0.001). The postoperative gastrointestinal function recovery time in the LF group was significantly shorter than in the OF group, and the difference had statistical significance, as shown in Figure 7.
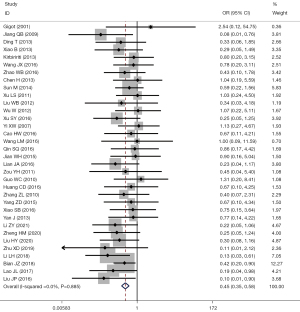
Meta-analysis of postoperative complications
A total of 34 studies reported problems related to the recovery time of postoperative gastrointestinal function, I2=0.0%, using the fixed-effects model for comparison between the two groups (OR =0.45, 95% CI: 0.35 to 0.58, P<0.001). The incidence rate of postoperative complications in the LF group was significantly less than that in the OF group, and the difference had statistical significance, as shown in Figure 8.
Sensitivity analysis
The sensitivity analysis results suggested there was no significant heterogeneity (Figure 9).
Funnel plot
A funnel plot was constructed to detect publication bias, and as it was basically left-right symmetrical, the results had no significant publication bias, as shown in Figure 10.
Discussion
Non-infectious hepatic cysts have an incidence of about 5% and the traditional treatment for symptomatic or large cysts are puncture and aspiration, cyst fenestration, cyst internal drainage, and cyst resection (51,52). Cyst aspiration is minimally invasive, but the postoperative recurrence rate is as high as 100% (53). OF of hepatic cysts is effective because the surgeon can complete manipulation under direct vision, but it is invasive and patients’ postoperative recovery is slow. Since hand-held laparoscopic treatment of non-parasitic hepatic cysts was reported in 1991, LF of hepatic cysts has gradually become one of the main methods of treatment (54). With the continuous improvement of laparoscopic techniques and instruments, current laparoscopic treatment of hepatic cysts has the advantages of minimal trauma, small scar, mild pain, exact effect, rapid recovery and low recurrence rate, and has become the first choice (55,56).
In this study, operation time, intraoperative blood loss, hospital stay, gastrointestinal function recovery time and postoperative complications of LF versus OF were analyzed. Compared with the laparotomy group, laparoscopic group had shorter operation time, less intraoperative blood loss, shorter hospital stay, faster postoperative recovery of gastrointestinal function and less postoperative complications. Sensitivity analysis demonstrated that the heterogeneity of the results was small, and the funnel plot revealed no significant publication bias. The number of included patients was large, making the results more reliable in showing that laparoscopic treatment can reduce pain and promote faster recovery of patients compared with the OF group, with higher efficacy and safety. The results of this study confirm the dominant position of laparoscopic surgery in modern surgery.
Study limitations: (I) all but two of the included studies were Chinese, so whether the same conclusion can be drawn for European and American populations still needs further study; (II) the randomized controlled trial methods were mostly unclear. The overall quality of the literature was uneven, with no high-quality original studies; (III) the conditions, age, sex and geographic location of the included study subjects differed, which may have led to a selection bias.
Conclusions
The clinical efficacy and safety of laparoscopic hepatectomy as treatment for giant hepatic cysts were superior to open hepatectomy. However, the conclusions still need to be further confirmed by more reliable studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-910/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-910/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu J, Zhang G, Gao Z, et al. Comparative analysis of laparoscopic fenestration with pedicand open treatment of giant liver cyst. The Journal of Laparoscopic Surgery 2016;21:108-11.
- Caremani M, Vincenti A, Benci A, et al. Ecographic epidemiology of non-parasitic hepatic cysts. J Clin Ultrasound 1993;21:115-8. [Crossref] [PubMed]
- Zacherl J, Scheuba C, Imhof M, et al. Long-term results after laparoscopic unroofing of solitary symptomatic congenital liver cysts. Surg Endosc 2000;14:59-62. [Crossref] [PubMed]
- Sun X. Discussion on the surgical treatment of non-parasitic giant liver cysts. Pharmaceutical Forum Journal 2011;32:106-7.
- Gloor B, Ly Q, Candinas D. Role of laparoscopy in hepatic cyst surgery. Dig Surg 2002;19:494-9. [Crossref] [PubMed]
- Katkhouda N, Mavor E. Laparoscopic management of benign liver disease. Surg Clin North Am 2000;80:1203-11. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Bian J. Efficacy and safety analysis of laparoscopic and open abdominal surgery in the treatment of liver cysts. Capital Food and Medicine 2018;25:32.
- Cao H, Jia C, Jiang Z. Clinical effect of laparoscopic fenestration in the treatment of liver cyst. Tibetan Medicine 2016;37:8-9.
- Chen H. Comparison of the clinical efficacy of laparoscopic fenestration and open abdominal fenestration in the treatment of liver cysts. The Clinical Journal of the Practical Hospital 2013;10:183-4.
- Chen J. Analysis of the clinical effect of laparoscopic fenestration drainage for the treatment of liver cyst. Inner Mongolia Medical Journal 2015;47:1351-3.
- Chen J, Chen J, Lin H, et al. Analysis of the clinical efficacy of laparoscopic treatment of liver cysts. Contemporary Chinese Medicine 2011;18:178.
- Chen W, Wu S, Tan M. Comparative observation of laparoscopic and open abdominal fenestration of liver cyst. Guangdong Medicine 2006;27:1073-4.
- Ding T, Xia D, Kuang Y. Clinical experience of laparoscopic treatment of liver cysts. Progress in Modern General Surgery in China 2013;16:489-91.
- Ferrizado Ding Y. Comparative study of laparoscopic and fenestration of open abdominal liver cyst. The Chinese Journal of Minimally Invasive Surgery 2009;9:999-1001.
- Gall TM, Oniscu GC, Madhavan K, et al. Surgical management and longterm follow-up of non-parasitic hepatic cysts. HPB (Oxford) 2009;11:235-41. [Crossref] [PubMed]
- Gigot JF, Metairie S, Etienne J, et al. The surgical management of congenital liver cysts. Surg Endosc 2001;15:357-63. [Crossref] [PubMed]
- Guo W, Huang J, Yi X, et al. Analysis of laparoscopic and open surgery of congenital liver cyst. The Journal of Gastroenterology and Hepatology 2010;19:362-3.
- Huang CD. Clinical application of laparoscopic fenestration and drainage for the treatment of liver cysts. Journal of Mathematical Medicine 2016;29:1436-8.
- Jian WH. Observation of the clinical effect and postoperative recovery of laparoscopic treatment in 38 patients with liver cysts. Capital Food and Medicine 2015;37-8.
- Jiang Q, Cai J, Chen J, et al. Discussion on different surgical treatment methods of simple liver cyst. The Modern Hospital 2009;9:56-8.
- Kirbiririti Nurmaimaiti. Nurk hotwood. Clinical analysis of laparoscopic fenestration of hepatic cyst. Chinese Journal of Trauma and Disability Medicine 2013;21:136-7.
- Lao J. Comparison between laparoscopic treatment and open treatment of liver cysts. Guangxi Medical University, 2017.
- Li L, Ma P, Xu H. Comparison of the clinical efficacy of laparoscopic and open abdominal fenestration in the treatment of liver cysts. Practical Integration of Integrated Chinese and Western Medicine 2018;18:115-6.
- Li Z. A control study on the clinical efficacy and safety of laparoscopic fenestration drainage and open abdominal surgery for the treatment of liver cyst. The Journal of Practical Medical Technology 2021;28:1235-7.
- Lian J, Fu Y, Jiang B, et al. Efficacy and safety comparison of laparoscopic and open abdominal window drainage in the treatment of liver cysts. Zhejiang Trauma Surgery Department 2016;21:921-2.
- Liu H, Zhang L, Mao H. Contrare the effects of laparoscopic small incision and open surgery on patients with liver cysts. Practical Integrated Clinical Practice of Traditional Chinese and Western Medicine 2020;20:123-4.
- Liu W, Ge Y, Xu G, et al. Clinical analysis of 32 cases of liver cysts. The Journal of Hepatobiliary Surgery 2012;20:179-81.
- Liu YP. Efficacy of laparoscopic window drainage and traditional open abdominal treatment of liver cyst and its effect on body stress response. Huaihai Medicine 2019;37:261-3.
- Qin S, Zhang Z, Li J, et al. Comparison of laparoscopic small incision and traditional laparotomy for the treatment of liver cysts. Hepar 2016;21:387-9.
- Song S, Ding Z, Zou H. Comparison of the clinical efficacy of minimally invasive and traditional laparotomy in patients with liver cysts. Traditional Chinese National Health Medicine 2017;29:31-3.
- Sun M, Zeng S, Huang D, et al. Retrospective comparative study of three fenestration drainage for the treatment of elderly congenital liver cysts. The Journal of Hepatobiliary and Pancreatic Surgery 2014;26:5-8.
- Wang J. Observation of the efficacy of laparoscopic fenestration surgery on liver cyst. The Journal of Clinical Medical Literature 2016;3:2052-3.
- Wang L, Wang X, Zhao Y, et al. Clinical efficacy of laparoscopic decompression of liver cyst. The World Journal of Chinese Digestion 2016;24:267-71.
- Wu W. Clinical analysis of laparoscopic fenestration for the treatment of hepatic cysts. China Pharmaceutical Guide 2012;9:32-3.
- Xiao B, Gu G, Zeng F, et al. Comparative effect of Windows and opening of laparoscopic liver cyst. Chinese and foreign Medical Studies 2013;11:26-7.
- Xiao S, Zhang C, Xu J, et al. Scleroclerosis and laparoscopic and laparotomy fenestration for treatment of liver cysts. Clinical Medicine 2016;36:87-8.
- Xu L, Liu W. Two prospective controlled studies of fenestration of liver cysts. The Journal of Hepatobiliary and Pancreatic Surgery 2011;23:66-8.
- Xu S. Analysis of the therapeutic effect of laparoscopic fenestration on hepatic cyst. The Chinese Medical Guide 2016;14:59-60.
- Yan J, Wu B. Surgical treatment of liver cysts. Rural Health Service Management in China 2013;33:236-7.
- Yang Z, Chen B. Comparison of laparoscopic and open treatment of liver cyst. Jilin Medicine 2015;36:279.
- Yi X, Huang J, Guo W, et al. Analysis of the clinical efficacy of laparoscopic fenestration of hepatic cyst. Journal of North Sichuan Medical College 2007;22:236-8.
- Zhang Z, Cui Z, Zheng L, et al. Analysis of the clinical treatment in 158 cases of liver cysts. Progress in Modern General Surgery in China 2010;13:787-91.
- Zhao B, Wang H. Clinical observation on clinical treatment of laparoscopic fenestration drainage for liver cyst. Chinese Community Physician 2016;32:53-4.
- Zhao W. Comparison of the efficacy of laparoscopic fenestration drainage and open fenestration on liver cysts. Clinical Medicine 2016;36:80-1.
- Zheng H. Efficacy and safety analysis of laparoscopic and open abdominal surgery in the treatment of liver cysts. Chinese and Foreign Medical Care 2020;39:33-5.
- Zheng X, Zeng P. Analysis of the efficacy of laparoscopic fenestration for the clinical treatment of liver cysts. Jilin Medicine 2012;33:5623.
- Zhu X, Wan H. Comparison of clinical efficacy of laparoscopic and open drainage in the treatment of liver cysts. Journal of Clinical Rational Drug Use 2019;12:140-1.
- Zou Y, Ma T, Jia Q. Control study of laparoscopic and open abdominal fenestration for the treatment of liver cysts. Contemporary Chinese Medicine 2011;18:7-8.
- Chen X. chirurgery (M). Version 2. Beijing: The People’s Health Press, 2011:608-13.
- Liu H, Wang Y, Hu Y, et al. Analysis of interventional puncture and drainage and surgical drainage in patients with liver cysts. The Journal of Practical Liver Disease 2021;24:745-8.
- Gigot JF, Legrand M, Hubens G, et al. Laparoscopic treatment of nonparasitic liver cysts: adequate selection of patients and surgical technique. World J Surg 1996;20:556-61. [Crossref] [PubMed]
- Cai Z, Huang Z, Lou A, et al. Clinical investigation of laparoscopic fenestration for the treatment of congenital hepatic cyst. Anhui Medicine 2013;34:759-60.
- Macutkiewicz C, Plastow R, Chrispijn M, et al. Complications arising in simple and polycystic liver cysts. World J Hepatol 2012;4:406-11. [Crossref] [PubMed]
- Brozzetti S, Miccini M, Bononi M, et al. Treatment of congenital liver cysts. A surgical technique tailored through a 35-year experience. Ann Ital Chir 2013;84:93-8. [PubMed]
(English Language Editor: K. Brown)







