
A detailed analysis of lymph node recurrence in endometrial carcinoma
Introduction
Uterine cancer, the second most common gynecological cancer in China, accounted for 2.18×104 death in 2015 (1). Endometrial carcinoma (EC), constituting the majority of uterine cancer, has a fairly low recurrence rate (2), and the lymph node (LN) is one of the main sites of recurrence (3-5). LN recurrence (LNR) was seldomly discussed as a separate topic, and was usually incorporated into the comparison between ‘distant recurrence’ and ‘locoregional recurrence’ (3). Studies discussing the risk or protective factors of LNR remain limited in number (5). Our study is designed to discuss the incidence, distribution and risk factors of LNR, including the potential protective effect of lymphadenectomy. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2588/rc).
Methods
Study population and treatment
We retrospectively analyzed patients with EC in Peking University People’s Hospital, who underwent surgery between 2006 to 2021. Clinicopathological data such as the International Federation of Gynecology and Obstetrics (FIGO) 2009 stage, tumor diameter, histology, lymphovascular space invasion (LVSI), peritoneal cytology, lymphadenectomy and adjuvant therapy, were collected from the medical record system. Histology was classified by invasiveness into ‘endometrioid G1/2’, ‘endometrioid G3’ and ‘non-endometrioid’. Generally, patients with EC (I) <2 cm; (II) myometrial invasion (MI) <1/2; (III) endometrioid G1/2 would have a surgery without systemic lymphadenectomy, otherwise a staging surgery ± sentinel LN (SLN) biopsy would be adopted. Pelvic lymphadenectomy (PLND) involved external, internal and common iliac LNs, obturator LNs and deep inguinal LNs in our institution. Whether to perform para-aortic lymphadenectomy (PALND) depended on the range and pathological characteristics of the tumor, including intraoperative pathological findings of SLNs. Risk grouping for recurrence was in accordance with the guideline by the European Society of Gynaecological Oncology, the European Society for Radiotherapy and Oncology and the European Society of Pathology (ESGO/ESTRO/ESP) published in 2015 (6). Adjuvant therapy for EC included radiotherapy and chemotherapy, which were adopted generally according to patients’ risk stratification, with age, health status and economic condition included in the practice as well. Patients with no less than intermediate risk generally received radiotherapy. The dose and range of radiotherapy depended on clinicopathological parameters, such as cervical involvement, LVSI, LN metastasis (LNM), residual foci, etc. Patients with no less than high-intermediate risk generally received chemotherapy ± radiotherapy, with platinum plus taxane being the most common regimen. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Peking University People’s Hospital (No. 2020PHB013-01) and individual consent for this retrospective analysis was waived.
Follow-up and recurrence classification
Follow-up data were collected using medical record system, phone call and text message, including physical examination, ultrasonography, tumor marker, etc. CT and MRI scan were adopted if necessary. Follow-up was conducted every 3 months for the first 2 years, every 6 months for the next 3 years, and yearly since the fifth year. LNR was defined as recurrence in LN bearing area, e.g., pelvic sidewall, para-aortic area, consistent with the previous study (5), with/without recurrence of other patterns. Besides LNR, pelvic recurrence and peritoneal recurrence were adopted to refer to recurrence occurring in pelvic cavity and peritoneal cavity, e.g., recurrence in the vaginal cuff and on the surface of the liver. Distant recurrence referred to recurrence in distant organs or deep in the parenchyma of pelvic and abdominal organs, e.g., recurrence in the liver parenchyma. Recurrence-free survival (RFS) was defined as the time between surgery and the first recurrence, or death from any cause, whichever occurred first, or the last visit for patients alive without recurrence. Thus, for a cohort only composed of patients with LNR and patients without recurrence (‘test-LNR’) to explore risk factors of LNR, RFS is equivalent to LNR-free survival.
Statistical analysis
To explore possible risk/protective factors, univariable analysis and multivariable analysis were conducted by Kaplan-Meier analysis and Cox regression model. Mann-Whitney test was adopted to evaluate the distribution of continuous variables between groups while Chi-square test was adopted for categorized variables. All variables had an effective rate above 95% except for molecular subtype. Therefore, missing data were omitted from the analysis, while the molecular subtype was reported separately in Table S1. P<0.05 was considered statistically significant. Data were collected and analyzed by R 4.0.3.
Results
Overview of LNR in EC patients
A total of 792 patients with EC were included in this study, with a median follow-up of 47 [1–110] months. Clinicopathological data were presented in Table 1. Seventy-three (9.2%) patients experienced recurrence, with a 3-year RFS (3yRFS) of 98.2% and a 5yRFS of 97.2%. Excluding 14 patients with sites of recurrence unknown, 21 patients (35.6%) had LNR, and 38 (64.4%) had recurrence without LNR. Patients with LNR had a median RFS of 16 [4–39] months, with 76.2% (16/21) experiencing recurrence within 2 years (Table S1).
Table 1
| Clinicopathological characteristics | LNR† (n=21) | Recurrence without LNR (n=38) | No recurrence (n=733) |
|---|---|---|---|
| Age (years), median [range] | 57.0 [46–76] | 57.0 [23–77] | 56.0 [28–83] |
| Tumor diameter (cm), median [range] | 3.8 [0.8–11.0] | 3.5 [0.1–10.0] | 2.5 [0.1–16.0] |
| CA125 (U/mL), median [range] | 38.0 [5.0–3,060.0] | 23.4 [4.2–530.8] | 20.5 [4.5–1,296.0] |
| Stage, n (%) | |||
| I | 6 (1.0) | 16 (2.6) | 602 (96.5) |
| II | 1 (2.3) | 2 (4.7) | 40 (93.0) |
| III | 8 (9.2) | 9 (10.3) | 70 (80.5) |
| IV | 6 (25.0) | 11 (45.8) | 7 (29.2) |
| Histology, n (%) | |||
| Endometrioid G1/2 | 6 (1.0) | 17 (2.8) | 574 (96.1) |
| Endometrioid G3 | 7 (8.2) | 5 (5.9) | 73 (85.9) |
| Non-endometrioid | 8 (8.3) | 16 (16.7) | 72 (75.0) |
| LVSI, n (%) | |||
| Negative | 14 (2.1) | 25 (3.8) | 619 (94.1) |
| Positive | 7 (5.8) | 13 (10.8) | 100 (83.3) |
| Peritoneal cytology, n (%) | |||
| Negative | 13 (1.8) | 23 (3.2) | 676 (94.9) |
| Positive | 5 (9.4) | 14 (26.4) | 34 (64.2) |
| Chemotherapy, n (%) | |||
| No | 4 (0.9) | 8 (1.8) | 438 (97.3) |
| Yes | 17 (5.2) | 30 (9.2) | 280 (85.6) |
| Radiotherapy, n (%) | |||
| No | 13 (2.6) | 28 (5.7) | 454 (91.7) |
| Yes | 8 (5.7) | 10 (7.1) | 122 (87.1) |
| Lymphadenectomy, n (%) | |||
| None | 3 (2.5) | 7 (5.7) | 112 (91.8) |
| Biopsy only | 0 | 3 (3.5) | 82 (96.5) |
| PLND | 5 (4.3) | 8 (7.0) | 102 (88.7) |
| PPALND | 13 (2.9) | 20 (4.4) | 423 (92.8) |
| LNM, n (%) | |||
| No | 11 (1.5) | 27 (3.8) | 673 (94.7) |
| Yes | 10 (14.9) | 11 (16.4) | 46 (68.7) |
†, with/without recurrence in sites beyond LNs. EC, endometrial carcinoma; CA125, carbohydrate antigen 125; LVSI, lymphovascular space invasion; PLND, pelvic lymphadenectomy; PPALND, pelvic and para-aortic lymphadenectomy; LNM, lymph node metastasis; LNR, lymph node recurrence; LNs, lymph nodes.
Distinct from LNM mainly involving pelvic and para-aortic LNs, LNR seemed more extensive, with a detailed description in Table S1. Common sites of LNR were as follows: pelvic LNs (9/21), retroperitoneal LNs (not specific) (5/21), inguinal LNs (4/21), para-aortic LNs (3/21), mediastinal LNs (2/21), supraclavicular LNs (2/21), the others.
Only 33.3% (7/21) of patients with LNR experienced LN-only recurrence, while the others experienced combined recurrence, with LN + distant ± others being the most common combination (7/21) (Figure S1).
Risk factors of LNR in EC patients
A cohort consisting of patients with LNR and patients with no recurrence was defined as ‘test-LNR’ (n=740), in order to discuss risk factors of LNR, excluding recurrent cases without LNR or with unknown sites of recurrence.
Advanced stage, poor histology, LVSI, tumor diameter ≥2 cm, LNM and adjuvant chemotherapy were significantly related to LNR by univariable analysis, among which only advanced stage [hazard ratio (HR): 6.8, 95% confidence interval (CI): 2.3–20.1], larger tumor diameter (HR: 1.2, 95% CI: 1.0–1.4) and poor histology (G3 vs. G1/2: HR: 6.9, 95% CI: 1.9–25.0; non-endometrioid vs. G1/2: HR: 10.6, 95% CI: 3.0–37.4) were independent risk factors of LNR by multivariable analysis (Table 2).
Table 2
| Clinicopathological features | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| N | P value | HR (95% CI) | P value | ||
| FIGO stage | <0.001* | ||||
| I/II | 649 | Reference | |||
| III/IV | 91 | 6.8 (2.3–20.1) | 0.001* | ||
| LVSI | 0.004* | ||||
| Negative | 633 | – | – | ||
| Positive | 107 | ||||
| Tumor diameter (cm)† | 0.004* | ||||
| <2 cm | 289 | 1.2 (1.0–1.4) | 0.032* | ||
| ≥2 cm | 447 | ||||
| Histological subtype | <0.001* | ||||
| Endometrioid G1/2 | 580 | Reference | |||
| Endometrioid G3 | 80 | 6.9 (1.9–25.0) | 0.003* | ||
| Non-endometrioid | 80 | 10.6 (3.0–37.4) | <0.001* | ||
| Peritoneal cytology | <0.001* | ||||
| Negative | 689 | – | – | ||
| Positive | 39 | ||||
| LNM | <0.001* | ||||
| No | 684 | – | – | ||
| Yes | 56 | ||||
| Adjuvant radiotherapy | 0.080 | ||||
| No | 467 | – | – | ||
| Yes | 130 | ||||
| Adjuvant chemotherapy | <0.001* | ||||
| No | 442 | – | – | ||
| Yes | 297 | ||||
| Lymphadenectomy | 0.421 | ||||
| Yes | 626 | – | – | ||
| No | 114 | ||||
†, adopted into multivariable analysis as a continuous variable; *, P<0.05. LNR, lymph node recurrence; FIGO, the International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion; LNM, lymph node metastasis; HR, hazard ratio; CI, confidence interval.
LNM and LNR
LNM was significantly associated with LNR in univariable analysis, and among patients with LNR, 47.6% (10/21) exhibited LNM at initial treatment, defined as ‘LNMR’. In LNMR patients, 80.0% (8/10) had recurrent LNs related to metastatic LNs, e.g., left deep inguinal LNs metastasis → left inguinal LNs recurrence. However, 60.0% (6/10) of such LNMR patients had recurrent LNs beyond the regions of initial LNM, e.g., pelvic LNs metastasis → pelvic, para-aortic and inguinal LNs recurrence (Table S1).
Intriguingly, all of LNMR patients underwent systemic lymphadenectomy, with 80.0% (8/10) undergoing pelvic and para-aortic lymphadenectomy (PPALND) and 20.0% (2/10) undergoing PLND. However, 90.0% (9/10) of LNMR patients had recurrent LNs overlapping with the range of lymphadenectomy, suggesting possible failure of lymphadenectomy to eradicate tumor cells in the lymphatic system, even within the scope of this operation.
Effect of lymphadenectomy on LNR
Univariable analysis suggested lymphadenectomy was not a protective factor for LNR in EC patients (Table 2). Further multivariable analysis including lymphadenectomy and independent risk factors reached in Table 2 didn’t support the protective effect of lymphadenectomy for LNR (P=0.351), either. A Chi-square test stratified by LNM exhibited no correlation between lymphadenectomy and LNR for both LNM cases and non-LNM cases (P>0.999 and P=0.701, respectively, Fisher’s exact test).
No association was suggested between the scope of lymphadenectomy and LNR by univariable analysis (Figure 1). Since no LNR occurred to patients undergoing LN biopsy only, they were merged with patients not undergoing lymphadenectomy into one group to conduct another univariable analysis, yet still failing to exhibit an association between systemic lymphadenectomy and a decrease in LNR (P=0.300).
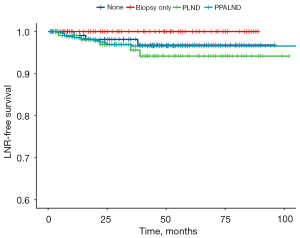
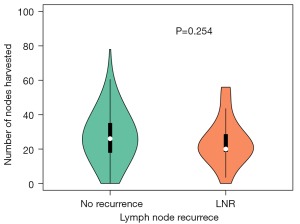
Median number of LNs harvested in LNR cases was 20 [0–56], while that of cases without recurrence was 26 [0–78] (Figure 2). No significant difference was observed between the groups (P=0.254, Mann-Whitney test).
Discussion
As the majority of uterine cancer, EC has a relatively low rate of recurrence (2,7), but the incidence of recurrence still indicates a poor prognosis (8). Recurrence is often detected in vagina, pelvic cavity, peritoneal cavity, lung, liver, bone, LN (pelvic, para-aortic), etc. (4,7,9). LNR, as one of the main patterns of EC recurrence (3,4), is seldom documented specifically (5). LNR indicates failure in the lymphatic system, and there might exist unique characteristics. We discussed several interesting topics of LNR, including its incidence, common sites, risk factors, as well as relationship between LNR and LNM, between LNR and lymphadenectomy.
LNR existed in over 1/3 of recurrent EC cases, with a more extensive pattern involving lymphatic system around the whole body (4,10,11). Still, LNs mostly involved in recurrent EC were those in metastatic cases at initial treatment, i.e., retroperitoneal LNs, suggesting a possible mechanism of LNR from growth of potential metastatic lesions in the lymphatic system, which survived primary surgery and adjuvant therapy. This was consistent with the univariable analysis identifying LNM as a risk factor for LNR. Several studies demonstrated a similar correlation (5,11). However, 88.9% of LNMR patients had recurrent LNs exceeding the range of LNM at initial treatment, suggesting that LNM might have the ability to further disseminate in the lymphatic network, rather than simply be an exhibition of invasiveness of the primary lesion. This research seems the first to describe this phenomenon in EC.
The benefit of lymphadenectomy in EC patients has been a controversial topic for over a decade (12-14). The survival advantage associated with lymphadenectomy exists primarily in patients with a relatively high risk of recurrence (14-18), and improperly expanding the indication of lymphadenectomy might result in more adverse effects instead (19-25). Our research didn’t support the role of lymphadenectomy in preventing LNR in terms of the scope and number of LNs harvest, with agreement with Mariani et al. (5). Lymphadenectomy removes LNs in certain node-bearing regions, instead of the whole lymphatic network. Considering the expanding property of the tumor in LNMR patients stated above, we propose chemotherapy agents with sufficient concentration and efficacy in lymphatic system might be crucial for prevention of LNR, rather than the attempt to eradicate LNs in drainage directions.
This study is the second study focused specifically on LNR in EC. In 2002, Mariani et al. firstly defined a similar concept, lymphatic failure, as a relapse occurring on node-bearing area as the primary site of failure. Different from our discussing risk factor in a cohort consisting of LNR and non-recurrence patients, Mariani et al. explored the difference between LNR and recurrence in other sites based upon a cohort of recurrent EC patients. Three predictors of lymphatic failure were LVSI, positive LNs, and cervical stromal invasion (5), two of which were also identified as risk factors of LNR in our research. Independent risk factors identified in our research, namely FIGO stage, tumor diameter and histology, were all related to malignant behavior of the tumor. Future research on the invasion, survival and proliferation of EC cells in the lymphatic system will help understand the formation of LNR and provide potential therapeutic targets.
Still there exist some limitations in our study. Over the span of 15 years, indications and practice of lymphadenectomy in EC have evolved in our institution, increasing the risk of confounding bias, e.g., random LN biopsy in the past vs. SLN biopsy nowadays. Also, due to relatively few cases of LNR, systematic comparison between LNR and recurrence in other sites as Mariani et al. was not conducted, and there was no separate discussion of cases with LN-only recurrence. Finally, a negative result might originate from a small sample size, rather than the objective lack of difference.
In summary, our study suggested LNR was common in patients with EC, with an extensive range and various patterns of recurrence. FIGO stage, tumor diameter and histology were independent risk factors of LNR, but lymphadenectomy seemed not protective for LNR.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81972426) and National Key Technology R&D Program of China (2019YFC1005200, 2019YFC1005201) to ZW.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2588/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2588/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2588/coif). ZW reports funding from National Natural Science Foundation of China (81972426) and National Key Technology R&D Program of China (2019YFC1005200, 2019YFC1005201). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Peking University People’s Hospital (No. 2020PHB013-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Simsek SY, Serbetcioglu G, Alemdaroglu S, et al. Clinicopathologic characteristics of recurrent endometrioid endometrial cancer patients and analysis of methods used durıing surveillance. J Gynecol Obstet Hum Reprod 2019;48:473-7. [Crossref] [PubMed]
- Iwase H, Furukawa S, Hirasawa T, et al. The Clinical Features of Recurrent Endometrial Cancer in Japan: Chemotherapy Instead of Radiotherapy as Postoperative Adjuvant Treatment. Int J Gynecol Cancer 2018;28:1616-23. [Crossref] [PubMed]
- Miyahara D, Yotsumoto F, Hirakawa T, et al. Clinical Features of Recurrence in Patients Without Residual Tumour in Endometrial Cancer. Anticancer Res 2019;39:4581-8. [Crossref] [PubMed]
- Mariani A, Webb MJ, Keeney GL, et al. Predictors of lymphatic failure in endometrial cancer. Gynecol Oncol 2002;84:437-42. [Crossref] [PubMed]
- Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol 2016;27:16-41. [Crossref] [PubMed]
- Francis SR, Ager BJ, Do OA, et al. Recurrent early stage endometrial cancer: Patterns of recurrence and results of salvage therapy. Gynecol Oncol 2019;154:38-44. [Crossref] [PubMed]
- Shim SH, Kim DY, Kim HJ, et al. Stratification of risk groups according to survival after recurrence in endometrial cancer patients. Medicine (Baltimore) 2017;96:e6920. [Crossref] [PubMed]
- Bricou A, Bendifallah S, Daix-Moreux M, et al. A Proposal for a Classification for Recurrent Endometrial Cancer: Analysis of a French Multicenter Database From the FRANCOGYN Study Group. Int J Gynecol Cancer 2018;28:1278-84. [Crossref] [PubMed]
- Montanelli L, Reato C, Mauro S, et al. A Rare Case Report: Isolated Mediastinal Lymph Node Recurrence in High-Risk Endometrial Cancer at 5 Years after Primary Laparoscopic Surgery. J Minim Invasive Gynecol 2018;25:537-40. [Crossref] [PubMed]
- Mahdi H, Jernigan A, Nutter B, et al. Lymph node metastasis and pattern of recurrence in clinically early stage endometrial cancer with positive lymphovascular space invasion. J Gynecol Oncol 2015;26:208-13. [Crossref] [PubMed]
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 2008;100:1707-16. [Crossref] [PubMed]
- ASTEC study group. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 2009;373:125-36. [Crossref] [PubMed]
- Todo Y, Kato H, Kaneuchi M, et al. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet 2010;375:1165-72. [Crossref] [PubMed]
- Yoon MS, Park W, Huh SJ, et al. Impact of paraaortic lymphadenectomy for endometrial cancer with positive pelvic lymph nodes: A Korean Radiation Oncology Group study (KROG 13-17). Eur J Surg Oncol 2016;42:1497-505. [Crossref] [PubMed]
- Eggemann H, Ignatov T, Kaiser K, et al. Survival advantage of lymphadenectomy in endometrial cancer. J Cancer Res Clin Oncol 2016;142:1051-60. [Crossref] [PubMed]
- Papathemelis T, Scharl S, Kronberger K, et al. Survival benefit of pelvic and paraaortic lymphadenectomy in high-grade endometrial carcinoma: a retrospective population-based cohort analysis. J Cancer Res Clin Oncol 2017;143:2555-62. [Crossref] [PubMed]
- Racin A, Raimond E, Bendifallah S, et al. Lymphadenectomy in elderly patients with high-intermediate-risk, high-risk or advanced endometrial cancer: Time to move from personalized cancer medicine to personalized patient medicine! Eur J Surg Oncol 2019;45:1388-95. [Crossref] [PubMed]
- Farrell R, Dixon SC, Carter J, et al. Lymphadenectomy in Early-Stage Intermediate-/High-Risk Endometrioid Endometrial Cancer: Clinical Characteristics and Outcomes in an Australian Cohort. Int J Gynecol Cancer 2017;27:1379-86. [Crossref] [PubMed]
- Papathemelis T, Hassas D, Gerken M, et al. Is there a benefit of lymphadenectomy for overall and recurrence-free survival in type I FIGO IB G1-2 endometrial carcinoma? A retrospective population-based cohort analysis. J Cancer Res Clin Oncol 2018;144:2019-27. [Crossref] [PubMed]
- Borghesi Y, Narducci F, Bresson L, et al. Managing Endometrial Cancer: The Role of Pelvic Lymphadenectomy and Secondary Surgery. Ann Surg Oncol 2015;22:S936-43. [Crossref] [PubMed]
- Coronado PJ, Rychlik A, Martínez-Maestre MA, et al. Role of lymphadenectomy in intermediate-risk endometrial cancer: a matched-pair study. J Gynecol Oncol 2018;29:e1. [Crossref] [PubMed]
- Bougherara L, Azaïs H, Béhal H, et al. Does lymphadenectomy improve survival in patients with intermediate risk endometrial cancer? A multicentric study from the FRANCOGYN Research Group. Int J Gynecol Cancer 2019;29:282-9. [Crossref] [PubMed]
- Li L, Tang M, Nie D, et al. Para-aortic lymphadenectomy did not improve overall survival among women with type I endometrial cancer. Int J Gynaecol Obstet 2020;150:163-8. [Crossref] [PubMed]
- Candido EC, Rangel Neto OF, Toledo MCS, et al. Systematic lymphadenectomy for intermediate risk endometrial carcinoma treatment does not improve the oncological outcome. Eur J Obstet Gynecol Reprod Biol X 2019;3:100020. [Crossref] [PubMed]


