
Complete response by patients with advanced hepatocellular carcinoma after combination immune/targeted therapy and transarterial chemoembolization: two case reports and literature review
Introduction
Hepatocellular carcinoma (HCC) leads to the third highest annual cancer-related mortality rate worldwide (1). Clinical manifestations of HCC usually do not appear until the middle or late stages of disease progression. As many as half of patients are first diagnosed when the cancer is already at an advanced stage. The preferred treatment for early-stage HCC is surgical resection, while effective treatment for advanced HCC is still lacking (2).
In recent years, the emergence of many new immunotherapies, such as immune checkpoint blockade therapy, has improved the treatment of advanced HCC (3). In particular, inhibitors targeting the interaction between T-cells and tumor cells mediated by the receptor programmed death (PD)-1 and its ligand PD-L1 have received attention. However, immune checkpoint inhibitors may not always be effective, reflecting the complex relationship between the tumor microenvironment and the immune response, which highlights the need to clarify under what circumstances such therapy works best (4). Combining PD-1/PD-L1 inhibitors with molecularly targeted drugs may drastically improve treatment of advanced HCC (5,6). Several strategies are already under investigation: the “dual immune regimen” combining Nivolumab with Ipilimumab, the “cola combination” combining Pembrolizumab with Lenvatinib, and the “A+T regimen” combining Atezolizumab with Bevacizumab. These combinations have improved the objective response rate of advanced HCC. It may also be possible to increase the efficacy of immunotherapeutic agents synergistically by first performing transarterial chemoembolization (TACE) (7), which can induce the release of large amounts of tumor antigens (8). This may improve patient outcomes and prognosis.
Here we report two patients diagnosed with advanced unresectable HCC who underwent local treatment modalities, such as TACE and radiofrequency ablation, and then achieved CR after combining immune and molecular targeted-therapies. Furthermore, we summarize the relevant literature on combination therapies to improve the clinical understanding of HCC and provide a basis for clinical treatment plan. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2691/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
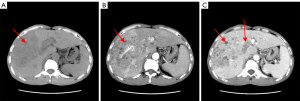
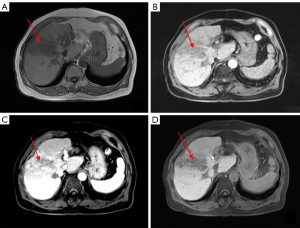
On February 20, 2020 a 47-year-old Chinese man was admitted to our hospital complaining of right upper abdominal pain for more than 10 days. The patient had a previous history of Hepatitis B and had no family history of HCC. Physical examination: light pressure pain in the right upper abdomen, failure to palpate the liver, and the rest of the physical examination was unremarkable. Blood was taken and the following parameters were measured: alpha-fetoprotein (AFP), 388.89 ng/mL; hepatitis B DNA, 2.99×105 IU/mL; total bilirubin, 33.9 µmol/L; albumin, 34.8 g/L; prothrombin time, 13.3 sec; and Child-Pugh liver function, A. Ultrasonography of the abdomen showed a lesion in parenchyma on the right lobe of the liver (Figure 1). Computed tomography (CT) of the upper abdomen showed a low-density mass in the right lobe of the liver, with an area of 8.6×13.3 cm2, and enhanced CT showed a “fast-in/fast-out” pattern, with extensive filling defect areas in the left and right branches of the portal vein and trunk (Figure 2A-2C).
The patient was diagnosed with HCC in Barcelona Clinic Liver Cancer (BCLC) stage C. The patient was treated with a combination of Lenvatinib (8 mg QD) and anti-PD-1 drugs (1 cycle every 21 days). After two cycles of targeted therapy and immunotherapy, the AFP level increased to 954.87 ng/mL. CT of the upper abdomen showed a slightly reduced hypodense mass in the right lobe of the liver, with an area of about 7.2×12.4 cm2; the left and right branches of the portal vein and the main trunk showed a slightly reduced area of filling defect (Figure 3A-3C). After four cycles of immunotherapy, the AFP level fell to 19.07 ng/mL, and the hypodense mass shrank to 5.2×9.2 cm2; the filling defect area of the left and right branches of the portal vein and the trunk was smaller and less dense than before (Figure 3D-3F). After six cycles of therapy, upper abdominal CT showed lesion in the right lobe of the liver measured 5.3×8.0 cm2 (Figure 3G-3I). After eight cycles, the AFP level was lower still, at 10.14 ng/mL, as was the lesion, at 4.2×6.3 cm2 (Figure 3J-3L). Throughout, the patient’s liver function remained at Child-Pugh grade A.
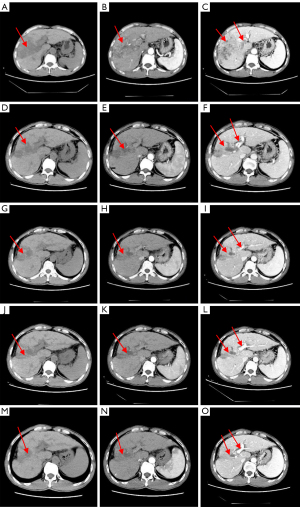
After a comprehensive analysis of the patient’s condition, TACE was performed, after which the patient underwent another four cycles of targeted therapy and immunotherapy. In the end, the patient’s AFP level dropped to 7.48 ng/mL, the lowest level since admission, and CT of the upper abdomen showed a lesion in the right lobe of the liver measuring only 3.8×3.6 cm2 (Figure 3M-3O). No active tumor lesion was found by embolization. We have used a timeline figure to summarize the clinical characteristics, treatment and prognosis of the patient in case 1 (Figure 4).
Case 2
On November 7, 2016, a 67-year-old Chinese man was hospitalized at Guangxi Medical University Cancer Hospital with right upper abdominal discomfort that had lasted more than 20 days. The patient had a previous history of Hepatitis B and had no family history of HCC. Physical examination: light pressure pain in the right upper abdomen, failure to palpate the liver, and the rest of the physical examination was unremarkable. Laboratory tests found AFP of 7.7 ng/mL, undetectable hepatitis B DNA, total bilirubin of 14.9 µmol/L, albumin of 44.3 g/L, prothrombin time of 10.9 sec, and Child-Pugh liver function grade of A. Abdominal ultrasonography showed a substantial lesion in the liver (Figure 5), and magnetic resonance imaging of the liver showed an abnormal signal mass in the left outer lobe of the liver and an abnormal signal nodule in the right lobe of the liver. The larger mass in the left lobe was 6.4×3.3 cm2. Enhanced CT showed “fast-in/fast-out”; the right branch of the portal vein was close to the mass, while the right branch of the portal vein was invaded (Figure 6).
The patient was diagnosed with HCC in BCLC stage C. At one week after admission, the patient was treated with TACE, followed one week later by radiofrequency ablation. After these interventions, the liver lesion measured 5.5×2.8 cm2 and showed a dense shadow of iodine oil deposition; the swelling shadow in the right posterior lobe of the liver was larger than at admission, measuring 4.5×3.8 cm2 (Figure 7). Hepatectomy was performed on the left lobe of the liver at two weeks after admission.
Postoperative pathology showed a liver mass with nodular cirrhosis and extensive nodular necrosis covering an area of 6×3.5×3 cm3, with clear borders. No residual tumor tissue, definite vascular carcinoma thrombi or satellite nodules were observed. The resection edge was negative for tumor cells. Immunohistochemistry failed to detect CK7, CK19, HBsAg or HBcAg; glypican-3 reactivity was borderline; and Ki67 expression was around 5%. Tissue was positive for p53. The pattern of CD34 expression indicated normal distribution of blood vessels.
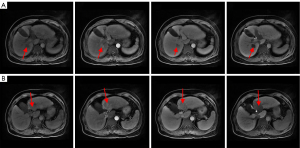
From 2017 to 2020, the patient returned to the hospital for regular bi-monthly check-ups, and no significant elevation of AFP was observed. During this period, however, the patient experienced multiple recurrences of intrahepatic lesions and was treated by radiofrequency ablation and anhydrous alcohol injection. Multiple new foci were seen at the junction of the left and right lobes, of which the largest measured 7.8×4.6 cm2. Additionally, the right anterior branch of the portal vein showed cancerous thrombosis (Figure 8A-8D).
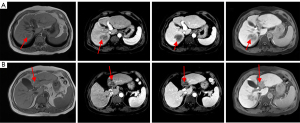
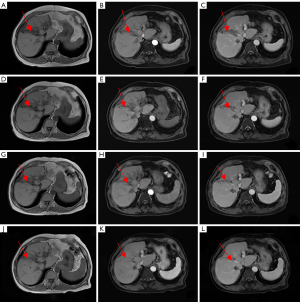
Combination therapy with Lenvatinib (8 mg QD) and anti-PD-1 drugs (1 cycle every 21 days) was initiated on July 17, 2020. After 3 cycles of targeted therapy and immunotherapy, AFP was 2.37 ng/mL and MRI of the liver showed shrinkage of the recurrent foci at the junction of the left and right lobes of the liver; the larger focus in the junction of the left and right lobes measured approximately 6.8×3.1 cm2 (Figure 9A-9C). After six and nine cycles of therapy, the recurrent foci at the junction of the left and right lobes of the liver continued to shrink, based on MRI (Figure 9D-9I). After 12 cycles, AFP was 2.27 ng/mL and MRI showed complete regression of the intrahepatic lesions (Figure 9J-9L). We have used a timeline figure to summarize the clinical characteristics, treatment and prognosis of the patient in case 2 (Figure 10).
Discussion
HCC has an insidious onset and most patients have no clinical symptoms. Patients with advanced HCC show median survival times of only 3.0 to 5.7 months, depending on whether they have hepatic macrovascular invasion, extrahepatic metastases, or both. The 1-year survival rate is only 12.0% to 38.3% (9-14). The lack of effective therapeutic measures for advanced HCC often means very poor prognosis, but immune checkpoint inhibitors combined with anti-angiogenic targeted drugs have recently become popular for treating unresectable advanced HCC and may improve outcomes. Our cases illustrate that such therapy may be particularly effective if performed after TACE.
PD-1 is an immunosuppressive molecule strongly expressed on tumor-specific T cells (15), while PD-L1 is expressed on tumor cells as an “immune-adaptive mechanism” to evade anti-tumor responses (16). When PD-1 is activated by its ligand, PD-L1, it initiates a series of responses that suppress T-cell activity and promote tumor cell growth (17), PD-1/PD-L1 inhibitors are drugs that block the interaction of PD-1 and PD-L1, restoring T-cell activity and the individual immune system’s ability to kill tumor cells (18). Several PD-1 inhibitors have shown efficacy in clinical trials, including Nivolumab (19), approved by the US Food and Drug Administration; Pembrolizumab (20); and Camrelizumab, which has been approved by the Chinese National Medical Products Administration (21).
Sorafenib and Lenvatinib are molecularly targeted anti-angiogenic drugs that prolong the survival of patients with advanced HCC. While sorafenib has historically been the only systemic drug widely used worldwide against advanced disease (22), Lenvatinib has recently emerged as a potentially better alternative (23,24); the drug has shown strong therapeutic effects against not only HCC tumors but also portal vein cancer thrombosis.
Nevertheless, monotherapies with either immunotherapeutic agents alone or molecularly targeted drugs alone are not entirely satisfactory (6). Combining the two approaches has shown the potential to achieve synergistic effects in several clinical trials, such as the combination of Lenvatinib and Pembrolizumab (25), Lenvatinib and Nivolumab (26), Camrelizumab and Apatinib (27), Atezolizumab and Bevacizumab (28), Sintilimab and Bevacizumab (29).
TACE can induce the release of local inflammatory factors and neoantigens (8), which induces a large number of T cells to migrate to the lesion site. This results in high expression of PD-1 molecules on the surface of the T cells, providing more targets for PD-1 inhibitors (30,31). Thus, several trials are evaluating the combination of TACE with immune checkpoint inhibitors. One trial, for example, found that combining TACE with Camrelizumab led to Objective Response Rates above 50% after the therapy (32). Ongoing are a multicenter, nonrandomized, pilot study (NCT03143270) on the safety and feasibility of drug-loaded microspheres for TACE in combination with Nivolumab (33), as well as a phase I/II trial on safety and efficacy of TACE followed by the PD-1 inhibitor Pabrolizumab (34). Radiofrequency ablation can also release neoantigens in a similar manner as TACE (35), and therefore we applied both TACE and ablation to our patients.
Given the heterogeneity of HCC, the combination of TACE and immune/targeted therapies that works best likely depends on individual patient characteristics. We suspect that the relatively good outcomes in our two cases reflect the fact that both were treated with TACE, which potentiated anti-PD-1 therapy; both showed high PD-1 expression, which is associated with better response to anti-PD-1 therapy (36); and both exhibited Child-Pugh A liver function, which may also be associated with better response to anti-PD-1 therapy (37). Interestingly our cases showed low or negligible AFP levels, suggesting that this level may not correlate with prognosis.
TACE may not be suitable for patients whose HCC involves portal vein trunk invasion because of the possibility of liver failure (38). Although both our patients showed such invasion, they also showed good blood flow and abundant collateral circulation, which may have contributed to good outcomes. These experiences are consistent with a previous report that TACE can be safe and effective in the presence of abundant collateral circulation around the portal vein (38).
During the treatment process, we continuously use the treatment modalities that promote the release of tumor antigens, such as TACE, radiofrequency ablation and other local treatments, so that the tumor antigens can be released as much as possible to strengthen the effect of immune and targeted therapy. We speculate that patients with advanced HCC with Child-Pugh grade A liver function may have better efficacy if they are treated with TACE and radiofrequency ablation first, followed by tumor necrosis and release of intratumor antigens to enhance the effect of immune and targeted therapy, and then sequential application of PD-1 inhibitors combined with molecular targeted drugs to transform therapy. stimulate the body immunity, so that patients may reach CR.
There are still shortcomings in this study: (I) pathological examination without surgical resection could not clarify whether pathological CR was achieved. (II) The future prognosis remains to be further observed. Although the patient achieved clinical CR with PD-1 inhibitor combined with Lenvatinib after TACE and radiofrequency ablation treatment, the duration of this treatment modality to maintain the patient’s CR efficacy and the duration of continued use of targeted and immunologic drugs after achieving clinical CR are still not clear. They are still in the stage of clinical exploration and need long-term observation and research.
In summary, the treatment of advanced HCC adopts a multi-modal combination therapy model, and individualized treatment plans should be developed according to individual conditions to improve the quality of life and maximize patient benefit. We report two cases of HCC patients who received TACE and radiofrequency ablation followed by sequential application of PD-1 inhibitors combined with molecularly targeted drugs for conversion therapy and achieved clinical CR. With these two cases, we speculate that for patients with advanced HCC who have Child-Pugh grade A liver function, lesions confined to the hemi-hepatic, tumors that are diffuse but fused into a single lesion, abundant peritumor blood flow, and portal vein carcinoma thrombosis staging Patients with advanced HCC not exceeding type III, after receiving TACE therapy and radiofrequency ablation, intratumoral antigens are released to achieve intensive immune and targeted therapy, and then PD-1 inhibitor combined with Lenvatinib conversion therapy is given with potentially better efficacy. At the same time, we caution that we did not perform histopathology on surgical specimens to determine whether pathological CR was achieved in our patients, nor did we assess outcomes in the long term. Future studies should also aim to optimize timing and duration of the different modalities in the combination therapy.
Acknowledgments
We would like to thank Chapin Rodríguez for his help in polishing our paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2691/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2691/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Song MJ. Hepatic artery infusion chemotherapy for advanced hepatocellular carcinoma. World J Gastroenterol 2015;21:3843-9. [Crossref] [PubMed]
- Jiang Y, Han QJ, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol 2019;25:3151-67. [Crossref] [PubMed]
- Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J 2018;24:47-53. [Crossref] [PubMed]
- Xie D, Sun Q, Wang X, et al. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med 2021 2021;9:652.
- Chen J, Hu X, Li Q, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients. Ann Transl Med 2020;8:1187. [Crossref] [PubMed]
- Palmer DH, Malagari K, Kulik LM. Role of locoregional therapies in the wake of systemic therapy. J Hepatol 2020;72:277-87. [Crossref] [PubMed]
- Yang F, Yang J, Xiang W, et al. Safety and Efficacy of Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma. Front Oncol 2022;11:657512. [Crossref] [PubMed]
- Cabibbo G, Enea M, Attanasio M, et al. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010;51:1274-83. [Crossref] [PubMed]
- Cabibbo G, Maida M, Genco C, et al. Natural history of untreatable hepatocellular carcinoma: A retrospective cohort study. World J Hepatol 2012;4:256-61. [Crossref] [PubMed]
- Xiang X, Zhong JH, Wang YY, et al. Distribution of tumor stage and initial treatment modality in patients with primary hepatocellular carcinoma. Clin Transl Oncol 2017;19:891-7. [Crossref] [PubMed]
- Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015;61:184-90. [Crossref] [PubMed]
- Yen YH, Cheng YF, Wang JH, et al. Real world clinical practice in treating advanced hepatocellular carcinoma: When East meets West. PLoS One 2020;15:e0230005. [Crossref] [PubMed]
- Mokdad AA, Singal AG, Marrero JA, et al. Vascular Invasion and Metastasis is Predictive of Outcome in Barcelona Clinic Liver Cancer Stage C Hepatocellular Carcinoma. J Natl Compr Canc Netw 2017;15:197-204. [Crossref] [PubMed]
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020;10:727-42. [PubMed]
- Ohaegbulam KC, Assal A, Lazar-Molnar E, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 2015;21:24-33. [Crossref] [PubMed]
- Buonaguro L, Mauriello A, Cavalluzzo B, et al. Immunotherapy in hepatocellular carcinoma. Ann Hepatol 2019;18:291-7. [Crossref] [PubMed]
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007;19:813-24. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571-80. [Crossref] [PubMed]
- Tovoli F, Negrini G, Benevento F, et al. Systemic treatments for hepatocellular carcinoma: challenges and future perspectives. Hepat Oncol 2018;5:HEP01. [Crossref] [PubMed]
- Takahashi K, Kim J, Takahashi A, et al. Conversion hepatectomy for hepatocellular carcinoma with main portal vein tumour thrombus after lenvatinib treatment: A case report. World J Hepatol 2021;13:384-92. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol 2020;38:2960-70. [Crossref] [PubMed]
- Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol 2020;38:4317-45. [Crossref] [PubMed]
- Yuan G, Cheng X, Li Q, et al. Safety and Efficacy of Camrelizumab Combined with Apatinib for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombus: A Multicenter Retrospective Study. Onco Targets Ther 2020;13:12683-93. [Crossref] [PubMed]
- D'Alessio A, Cammarota A, Zanuso V, et al. Atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma. Expert Rev Anticancer Ther 2021;21:927-39. [Crossref] [PubMed]
- Liu W, Quan B, Lu S, et al. First-Line Systemic Treatment Strategies for Unresectable Hepatocellular Carcinoma: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Front Oncol 2021;11:771045. [Crossref] [PubMed]
- Shi L, Chen L, Wu C, et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin Cancer Res 2016;22:1173-84. [Crossref] [PubMed]
- Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014;211:781-90. [Crossref] [PubMed]
- Zhang S, Zhao Y, He L, et al. Effect of camrelizumab plus transarterial chemoembolization on massive hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2022;46:101851. [Crossref] [PubMed]
- Leone P, Solimando AG, Fasano R, et al. The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment. Vaccines (Basel) 2021;9:532. [Crossref] [PubMed]
- Singh P, Toom S, Avula A, et al. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma 2020;7:11-7. [Crossref] [PubMed]
- Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013;57:1448-57. [Crossref] [PubMed]
- Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 2019;18:10. [Crossref] [PubMed]
- Spahn S, Roessler D, Pompilia R, et al. Clinical and Genetic Tumor Characteristics of Responding and Non-Responding Patients to PD-1 Inhibition in Hepatocellular Carcinoma. Cancers (Basel) 2020;12:3830. [Crossref] [PubMed]
- Kim JH, Yoon HK, Kim SY, et al. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther 2009;29:1291-8. [Crossref] [PubMed]






