
Anlotinib improved the reactive cutaneous capillary endothelial proliferation induced by camrelizumab: a case report
Introduction
In recent years, especially since 2018, the China National Medical Products Administration (NMPA) has successively approved a variety of immune checkpoint inhibitors (ICIs) targeted to programmed cell death protein-1 (PD-1) or its ligand PD-L1. These monoclonal antibodies have opened a new era of tumour immunotherapy and have significantly improved the overall survival of many patients with advanced solid tumours (1,2). Camrelizumab (SHR-1210) is a PD-1 monoclonal antibody that was developed independently by Chinese scholars and national pharmaceutical enterprises. On 31 May 2019, the NMPA approved it for the treatment of classical Hodgkin lymphoma, hepatocellular carcinoma (HCC), non-small cell lung cancer (NSCLC), and oesophageal squamous cell carcinoma. In China, camrelizumab is the ICI with the most approved indications and a wide range of clinical applications (3).
Similarly to other anti-tumour drugs, PD-1 inhibitors can lead to a clear therapeutic effect alongside unique adverse reactions related to their mechanism. Because of drug-mediated over activation of the immune system, ICIs may result in immune-related adverse events (irAEs) (4). The most common adverse reaction of camrelizumab is reactive cutaneous capillary endothelial proliferation (RCCEP), an immune-related and self-limiting condition (5). Due to limited data, the exact pathophysiological mechanism of RCCEP is still unclear, and its appearance may be due to the imbalance between angiogenesis promoters and inhibitors. Anlotinib is a small molecule inhibitor of multiple receptor tyrosine kinases; it has broad-spectrum inhibitory effects on angiogenesis and tumour growth through inhibition of vascular endothelial growth factor receptor (VEGFR) 2 and 3 (6). Some reports indicate that combination of anlotinib and anti-PD-1 antibodies demonstrated promising durable antitumor efficacy with hypotoxic in patients with various advance tumors (7-9). Anlotinib could be a potential management to reduce the adverse reactions that are treated with camrelizumab. Therefore, it is particularly important to improve clinical awareness of this specific skin toxicity and monitor which medications lead to this condition. This endeavour will allow clinicians to identify the best treatment time and provide timely symptomatic measures for patients in subsequent research. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-426/rc).
Case presentation
In April 2021, a 57-year-old man was presented with a cough and expectoration without an obvious remote cause. Bronchoscopy was performed and the pathology results showed squamous cell carcinoma. The chest computed tomography (CT) scan also revealed a soft tissue mass at the bronchial opening of the upper lobe of the left lung with invasion of the left pulmonary artery. He was diagnosed with left upper central lung cancer with metastasis to multiple lymphocytes in the mediastinum and left hilum of the lung. The image staging was T4N1. PD-1 test results showed tumor proportion score (TPS) =5% and combined positive score (CPS) =6. The patient received treatment with paclitaxel (albumin bound) 300 mg plus camrelizumab 200 mg and recombinant human endostatin on 27 May 2021. Later, he received chemotherapy [paclitaxel (albumin bound) 200 mg days 1 and 8; cisplatin 40 mg days 1–3] combined with immunotherapy (camrelizumab 200 mg) on 23 June, 15 July, and 9 August 2021. He had achieved partial response after four cycles of chemotherapy combined with immunotherapy (Figure 1). He was admitted to our hospital for his fifth cycle on 31 August 2021.
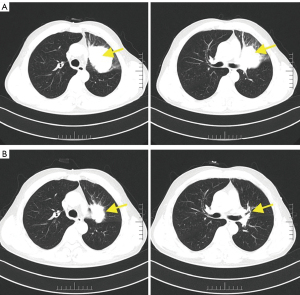
His admission diagnosis was: (I) left upper lobe squamous cell carcinoma after chemotherapy and immunotherapy, with a clinical stage of T4N2M1c, IVB; (II) mediastinal lymphocytes metastasis; and (III) hepatic metastases. The relevant examinations were performed in an effort to eliminate treatment-related contraindications. He was then treated with camrelizumab 200 mg on 4 September 2021. The chemotherapy plan was paclitaxel (albumin bound) 200 mg days 1 and 8 plus cisplatin 40 mg days 1–3 beginning on 6 September 2021. On 8 September 2021, physical examination revealed that his face, head, neck, and chest skin had multiple scattered bright red round papules, especially his head and neck. That features were ‘red-nevus-like’ and ‘pearl-like’, and the maximum diameter was about 6 mm. There was a rash under the left eye that had ruptured and was bleeding (Figure 2). After dermatological consultation, it was diagnosed as RCCEP. No special treatment was performed locally. It was recommended that the patient avoid scratching or rubbing. Mupirocin ointment was used for symptomatic support to prevent skin and soft tissue infections at the ulcer. The patient was treated with oral anlotinib hydrochloride capsule 8 mg once a day. On 12 September 2021, the patient’s rash improved significantly, the papule became lighter and the size atrophied, and no new RCCEP was found on other parts of the body. And after another 2 days treatment then anlotinib was discontinued.
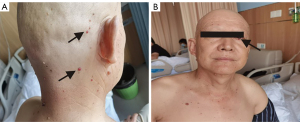
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Correlation evaluation
The instructions of the PD-1 inhibitor camrelizumab clearly indicate that RCCEP is the most common adverse reaction; it is common for many ICIs (10). In most patients, RCCEP appears in the first cycle during the first treatment (11). In a phase 1 clinical trial including 36 patients with advanced solid tumours, the median time to RCCEP occurrence was 23 days (12). In our case, however, RCCEP occurred 4 days after the fifth cycle of immunotherapy had commenced, a time that is different from the above-mentioned study. However, in another case report, two patients developed RCCEP later, mostly scattered bright red punctate papules on the skin during third and second cycles of camrelizumab, respectively (13).
Based on a prospective study, most patients achieved regression of RCCEP during or after treatment with camrelizumab (14). Song et al. (15). reported that in a phase 2 clinical trial of camrelizumab for the treatment of 76 patients with classic Hodgkin lymphoma, the incidence of RCCEP was 97.3%. The clinicopathological characteristics of RCCEP have been described in many guidelines and there is a consensus. RCCEP appears on the skin surface, mainly on the skin of the head, face, and trunk. The lesions were usually bright red spots: most lesions are ‘nodule-like’, a few lesions are ‘patch-like’, and some lesions have bright or dark red colouration. RCCEP can be divided into five types according to the morphology, namely, ‘red-nevus-like’, ‘pearl-like’, ‘mulberry-like’, ‘patch-like’, and ‘tumour-like’ (16,17). Our patient presented papules that are extremely similar to the guidelines and consensus. They were mainly ‘red-nevus-like’ and ‘pearl-like’, and they were considered to be RCCEP based on the dermatological consultation. No RCCEP-related symptoms have been reported in patients taking paclitaxel (albumin bound) or cisplatin. Of note, RCCEP is strongly related to camrelizumab treatment, denoted by a score of 6 on Naranjo’s assessment scale.
The possible mechanism of RCCEP induced by camrelizumab
ICIs have achieved significant outcomes in the treatment of a variety of malignancies (18). However, ICIs may result in irAEs due to excessive activation of immune system, and this issue has attracted great attention (19). irAEs commonly occur in the skin, colon, endocrine organs, liver, and lung, among other sites (20). While RCCEP is a common skin irAEs related to camrelizumab, the exact mechanisms by which is occurs are still under investigation. In a multicentric phase 2 clinical study (21), 145 camrelizumab-treated patients experienced RCCEP. Immunohistochemistry showed intense staining of endothelial cells (denoted by CD31) as well as proliferation and division of endothelial cells (denoted by Ki67). High expression of vascular endothelial growth factor-A (VEGF-A) and VEGFR2-pY1175 in the lesions indicated activation of angiogenesis via the VEGFR2 signalling pathway. Based on these findings, camrelizumab might activate CD4+ T cells, thus increasing Th2 release of the cytokine interleukin-4 (IL-4), stimulating the differentiation of CD163+ M2 macrophages, and then promoting vascular proliferation by releasing VEGF-A. This phenomenon would induce proliferation of skin capillary endothelial cells and eventually lead to RCCEP.
Effect of camrelizumab combined with targeted therapy on RCCEP
A summary analysis found that the incidence of RCCEP with camrelizumab monotherapy was 56.6%, whereas the incidence of RCCEP with camrelizumab plus chemotherapy or anti-vascular treatment decreased to 42.7% and 45.3% (22). The shortest median duration from onset to remission of RCCEP in the combined camrelizumab plus anti-vascular treatment was 2.2 months. A multicentric, open-label, single-arm, phase 2 trial showed that the incidence of RCCEP with camrelizumab combined with apatinib for treatment of 45 patients with advanced cervical cancer was 8.9% (23). Therefore, anti-angiogenic drugs are considered to decrease the occurrence of RCCEP caused by camrelizumab.
Anlotinib is a small molecule inhibitor of multiple receptor tyrosine kinases; it has broad-spectrum inhibitory effects on angiogenesis and tumour growth. Anlotinib suppresses tumour cell proliferation through inhibition of VEGFR 2 and 3, fibroblast growth factor receptors 1–4 (FGFR 1–4), platelet-derived growth factor receptors α/β (PDGFR α/β), c-Kit and Ret. In China, it is approved for the treatment of patients with locally advanced or metastatic NSCLC (6). The occurrence of RCCEP may be related to the activation of the VEGFR2 signalling pathway and other factors. Therefore, we speculate that the combined use of anlotinib may relieve RCCEP-related symptoms in patients.
Management of RCCEP caused by camrelizumab
The management of RCCEP caused by camrelizumab is based on guidelines and consensus (16,17). According to the grade and clinical symptoms of RCCEP, different management steps can be taken. Camrelizumab therapy can be continued while managing grade 1–2 RCCEP. The easily rubbed parts can be protected with gauze to avoid bleeding. If the rash ruptures and bleeding occurs, local haemostasis can be started to prevent infection, and other symptomatic treatment can be implemented. For RCCEP grade 3 or higher, camrelizumab therapy should be delayed. In a multicentric, randomised, open-label, phase 3 clinical study, the occurrence of RCCEP was related to the objective response rate of camrelizumab (24). The median survival of patients who had and did not have RCCEP was 10.1 and 2.5 months, respectively.
Pharmaceutical suggestions
Camrelizumab has only been approved in China since 2019, and its irAEs including RCCEP still need to be studied. Clinical pharmacists should master the clinical characteristics, morphological manifestations, drug correlation, corresponding treatment measures, and prognosis of RCCEP caused by camrelizumab. Appropriate medication education and preparing patients for potential side effects such as RCCEP must be performed to ensure the safety and effectiveness of medication.
Conclusions
In conclusion, we have reported a case of RCCEP induced by anti-PD-1 blockade via camrelizumab on day 4 of the fifth cycle of immunotherapy. The patient was given oral anlotinib to relieve the symptoms of RCCEP. The specific pathophysiological mechanism of RCCEP remains unclear. Activation of angiogenesis via the VEGFR2 signalling pathway might be the most persuasive hypothesis. The risk for RCCEP should always be kept in mind during camrelizumab treatment. It is necessary to determine the specific mechanism and predictive risk factors of RCCEP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-426/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-426/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-426/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou J, Mao Q, Li Y, et al. Oral reactive capillary hemangiomas induced by SHR-1210 in the treatment of non-small cell lung cancer: a case report and literature review. BMC Oral Health 2021;21:559. [Crossref] [PubMed]
- Tang B, Chi Z, Chen Y, et al. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Previously Treated Advanced Melanoma: Results of the POLARIS-01 Multicenter Phase II Trial. Clin Cancer Res 2020;26:4250-9. [Crossref] [PubMed]
- Chen Y, Zhang H, Shi J, et al. A Partial Response of Pulmonary Pleomorphic Carcinoma to Camrelizumab (PD1 Monoclonal Antibody) Monotherapy: A Case Report. Onco Targets Ther 2020;13:12471-6. [Crossref] [PubMed]
- Darnell EP, Mooradian MJ, Baruch EN, et al. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr Oncol Rep 2020;22:39. [Crossref] [PubMed]
- Zhang X, Wang M, Shi B. Reactive capillary hemangiomas induced by camrelizumab in a patient with hepatocarcinoma. Int J Dermatol 2021;60:e409-10. [Crossref] [PubMed]
- Gao Y, Liu P, Shi R. Anlotinib as a molecular targeted therapy for tumors. Oncol Lett 2020;20:1001-14. [Crossref] [PubMed]
- Yuan M, Zhu Z, Mao W, et al. Anlotinib Combined With Anti-PD-1 Antibodies Therapy in Patients With Advanced Refractory Solid Tumors: A Single-Center, Observational, Prospective Study. Front Oncol 2021;11:683502. [Crossref] [PubMed]
- Zhou N, Jiang M, Li T, et al. Anlotinib combined with anti-PD-1 antibody, camrelizumab for advanced NSCLCs after multiple lines treatment: An open-label, dose escalation and expansion study. Lung Cancer 2021;160:111-7. [Crossref] [PubMed]
- Wang P, Fang X, Yin T, et al. Efficacy and Safety of Anti-PD-1 Plus Anlotinib in Patients With Advanced Non-Small-Cell Lung Cancer After Previous Systemic Treatment Failure-A Retrospective Study. Front Oncol 2021;11:628124. [Crossref] [PubMed]
- Yu Q, Wang WX. Camrelizumab (SHR-1210) leading to reactive capillary hemangioma in the gingiva: A case report. World J Clin Cases 2020;8:624-9. [Crossref] [PubMed]
- Zhao J, Su C. Comments on CSCO management guideline of toxicities from immune checkpoint inhibitors: comparing with NCCN guideline. Journal of Practical Oncology 2020;35:11-5.
- Mo H, Huang J, Xu J, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer 2018;119:538-45. [Crossref] [PubMed]
- Wei B, Wang H, Liu F, et al. Reactive cutaneous capillary endothelial proliferation caused by the PD-1 inhibitor camrelizumab during the treatment of malignant tumors: two case reports. Chinese Journal of Dermatology 2021;54:252-3.
- Markham A, Keam SJ. Camrelizumab: First Global Approval. Drugs 2019;79:1355-61. [Crossref] [PubMed]
- Song Y, Wu J, Chen X, et al. A Single-Arm, Multicenter, Phase II Study of Camrelizumab in Relapsed or Refractory Classical Hodgkin Lymphoma. Clin Cancer Res 2019;25:7363-9. [Crossref] [PubMed]
- CSCO. Consensus on the clinical diagnosis and treatment of reactive skin capillary hyperplasia caused by carrelizumab. Chinese Clinical Oncology 2020;25:840-8.
- National Health Commission of the People's Republic of China. Guidelines for the clinical application of new anti-tumor drugs, 2019. Journal of Multidisciplinary Cancer Management 2020;6:16-47. (Electronic Version).
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020;6:38. [Crossref] [PubMed]
- Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. [Crossref] [PubMed]
- Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol 2020;13:47. [Crossref] [PubMed]
- Qu W, Wang F, Qin S. Retrospective study of reactive cutaneous capillary hyperplasia induced by carrelizumab. Proceedings of 2020 CSCO annual meeting: special session on innovative drugs 2020.
- Lan C, Shen J, Wang Y, et al. Camrelizumab Plus Apatinib in Patients With Advanced Cervical Cancer (CLAP): A Multicenter, Open-Label, Single-Arm, Phase II Trial. J Clin Oncol 2020;38:4095-106. [Crossref] [PubMed]
- Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832-42. [Crossref] [PubMed]


