
Usefulness of the estimation of physiologic ability and surgical stress (E-PASS) system for prediction of complication and prognosis in hepatocellular carcinoma patients after hepatectomy
Introduction
Globally, liver cancer ranks the sixth cancer incidence and is the fourth leading cause of cancer related-death (1). As the most common pathological type of liver malignant tumor, hepatocellular carcinoma (HCC) accounts for 70–80% of all cases (2). The prognosis of HCC patients depends on the tumor staging, benefits of interventions and individual’s physical characteristics (3,4). Surgeries, including hepatic resection and liver transplantation, are considered to be the backbone of curative treatment, especially in patients with early-stage HCC (5). Undoubtedly, most patients have to choose surgical resection because of the organ rejection and the shortage of available donors. However, around 90% of HCC patients developed in a background of liver cirrhosis, and the mortality of cirrhotic patients after surgical resection is estimated between 3 and 14% (6,7).
The purpose of surgical treatment is to improve the prognosis of HCC patients, so the risk of postoperative complications or death after hepatectomy deserves the attention of surgeons. Though remarkable development in surgical technique and perioperative management have significantly reduced postoperative mortality or morbidity in HCC patients undergoing hepatectomy, the incidence of postoperative complications after hepatectomy are still higher than other oncologic operations (8,9). Post-hepatectomy liver failure is a serious complication after hepatectomy and the leading cause of postoperative death in patients with incidence rates ranging from 0.7% to 34% (10). Other common but serious complications include hemorrhage, biliary fistula, pneumonia, ascites, abdominal abscess, organ dysfunction and so on. Therefore, it is extremely matters to evaluate and identify the high-risk patients after hepatectomy rapidly and intuitively.
In 1999, Haga et al. (11) proposed E-PASS system in predicting postoperative morbidity and mortality after gastrointestinal surgery. The E-PASS system took preoperative factors [Preoperative Risk Score (PRS)] and surgical factors [Surgical Stress Score (SSS)] into account, whose efficiency has been validated in many surgical operations, such as in gastrointestinal and pancreatic surgery (12-15). The duration of surgery and portal occlusion are also thought to be associated with the occurrence of complications after hepatectomy (16,17). Given that the E-PASS system comprehensively assesses individual’s physiological state and surgical stress, it may predict postoperative morbidity after hepatectomy. Banz et al. (18) reported that for patients undergoing liver resection due to various etiologies, the E-PASS system seems to be an effective predictor of postoperative mortality, but not suitable for postoperative complications. In another research (19), only PRS may be associated with systemic complications after hepatectomy.
Therefore, the purpose of this research was to verify whether the E-PASS system can predict occurrence of postoperative complication in HCC patients. Meanwhile, we explored the relationship between the E-PASS system and the long-term prognosis of such patients. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-352/rc).
Methods
Patients
We retrospectively analyzed patients who underwent hepatectomy for liver tumors in Sir Run Run Shaw Hospital, Zhejiang University from January 2018 to December 2019. The inclusion criteria were as follows: (I) the patient was diagnosed as primary HCC in postoperative pathological examination; (II) complete examination results, hospitalization records and follow-up records required for the study were available; (III) the age was more than 18 years old. The exclusion criteria were as follows: (I) the postoperative pathological diagnosis was other intrahepatic tumors or metastatic hepatic cancer; (II) undergone liver resection for any disease previously; (III) diagnosed as HCC preoperatively and suffered adjuvant therapy such as transarterial chemoembolization; (IV) data was incomplete or lost follow-up. The ways of follow-up were based on telephone connections and outpatient examinations. The last time of follow-up was September 2021 and the median fellow-up time was 27 months (range, 1 to 44 months). A total of 236 cases were eventually analyzed in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital of Zhejiang University, Hangzhou, China (No. 20210729-282). Individual consent for this retrospective analysis was waived.
Data collection
Baseline characteristics of patients, including age, gender, body mass index (BMI), chronic diseases. Preoperative laboratory data includes hemoglobin, lymphocyte, neutrophil, platelet, albumin, total bilirubin, alpha-fetoprotein (AFP), C-reactive protein (CRP) and hepatitis B virus surface antigen were completely collected. The estimated bleeding volume and operation time were obtained according to the operation notes. The cancer stage was determined according to the eighth edition of the American Joint Committee on Cancer (AJCC) and Barcelona Clinic Liver Cancer (BCLC) staging system (20,21). Postoperative complications were defined as complications that occurred before discharge or within 1 month after discharge and related to the surgery. Meanwhile, according to the Clavien-Dindo classification (22), complications of Clavien-Dindo grade II were defined as mild complications, while Clavien-Dindo grades III–IV were classified into major complications.
E-PASS system, prognostic nutritional index (PNI) and albumin-bilirubin index (ALBI)
The equations of the E-PASS system were developed from a previous study (11).
X1: age; X2: the absence [0] or presence [1] of severe heart disease; X3: the absence [0] or presence [1] of severe pulmonary disease; X4: the absence [0] or presence [1] of diabetes mellitus; X5: the performance status (PS) index (range, 0 to 4); X6: the American Society of Anesthesiologists (ASA) physiologic status classification (range, 1 to 5).
Severe heart disease is defined as heart failure meeting the NYHA Class III or IV or severe arrhythmia requiring mechanical support (23). Severe pulmonary disease is defined as any condition of vital capacity (VC) <60% and/or forced expiratory volume in 1 second (FEV1) <50%. Diabetes mellitus is defined according to the World Health Organization criteria (24). The PS index is defined according to the Japan Society for Cancer Therapy, which is similar to the Eastern Cooperative Oncology Group (ECOG) criteria (25): grade 0, without symptoms or restriction in social activities; grade 1, mild symptoms that restrict physically strenuous activity; grade 2, capable of all self-care but unable to carry out any activities; grade 3, in need of physical assistance for daily living; grade 4, requirement of constant physical assistance without any self-care ability. The ASA physiologic status classification: class 1, a healthy patient; class 2, a patient with mild systemic disease; class 3, a patient with severe systemic disease without life threat; class 4, a patient with severe systemic disease that is a threat to life; class 5, a patient who is expected to die in 24 hours with or without surgery (26).
X1 = estimated blood loss/body weight (mL/kg). X2 = operation time (h). X3 = extent of skin incision: laparotomy plus thoracotomy [2], laparotomy [1], laparoscopy [0].
The calculation formula of PNI:
The calculation formula of ALBI (27,28):
Statistical analysis
Continuous variables were presented as median (range) or mean ± standard deviation (SD) and analyzed by the Mann-Whitney U test or the Student’s t-test. Categorical variables were presented as numbers (percentage) and analyzed by the Pearson’s chi-square test or the Fisher’s exact test. Factors with statistically difference between the complication group and non-complication group were further included in the logistic regression analysis. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were constructed to select an optimal critical value according to the incidence of postoperative complications. Cox proportional hazards model was applied to analyze the impact of univariate or multivariate on overall survival (OS) and recurrence-free survival (RFS). Survival curve was analyzed by Kaplan-Meier method and log-rank test was used for further comparison. The inverse variance method was performed for dichotomous variables and the effect measure was odds ratio (OR) with 95% confidence interval (CI). Statistical difference was considered to be significant at P<0.05. The software tools of statistical analyses were conducted by SPSS 22.0 (Armonk, NY, USA: IBM Corp.).
Results
Characteristics of patients in two groups
A total of 236 patients diagnosed pathologically as primary HCC and underwent radical surgery were included in this study. According to the presence or absence of postoperative complications, these patients were divided into two groups. The baseline characteristics and preoperative laboratory data of each group are shown in Table 1. Seventy-nine (33.5%) patients suffered from postoperative complications after surgery while 157 (66.5%) did not. Most of baseline data and baseline characteristics including age, gender, BMI, concurrent diseases showed no significant differences between two groups. However, total bilirubin and ALBI were significantly different between two groups (19.0±8.3 vs. 15.3±6.9, P<0.001; −2.58±0.37 vs. −2.69±0.42, P=0.046, respectively). In E-PASS system, patients suffered postoperative complication had higher SSS and CRS [0.047 (−0.267 to 1.118) vs. −0.180 (−0.297 to 0.450), P<0.001; 0.169 (−0.351 to 1.159) vs. −0.128 (−0.421 to 0.686), P<0.001, respectively]. PRS showed no significant difference between complication group and non-complication group. Notably, maximum diameter of tumors was different in two groups, which was larger in patients with higher postoperative complications rate [4.5 (1.0–15.5) vs. 3.4 (0.7–15.0), P=0.012].
Table 1
| Characteristics | Complication (+) (n=79) | Complication (−) (n=157) | P |
|---|---|---|---|
| Age (years) | 61.0±11.4 | 59.1±10.8 | 0.210 |
| Gender (male/female) | 67/12 | 132/25 | 0.884 |
| Weight (kg) | 64.3±11.7 | 64.6±9.7 | 0.826 |
| BMI (kg/m2) | 23.1±3.3 | 23.2±2.9 | 0.868 |
| Hypertension (with) | 33 (41.8) | 50 (31.8) | 0.132 |
| Diabetes mellitus (with) | 18 (22.8) | 21 (13.4) | 0.066 |
| Hemoglobin (g/L) | 140.9±17.2 | 139.2±16.6 | 0.453 |
| Lymphocyte count (×109/L) | 1.5±0.7 | 1.6±0.7 | 0.702 |
| Neutrophils count (×109/L) | 3.1±1.2 | 3.4±1.4 | 0.061 |
| Platelet (×109/L) | 142.1±65.8 | 149.7±56.7 | 0.385 |
| Albumin (g/L) | 40.0±4.5 | 40.6±5.0 | 0.374 |
| Total bilirubin (μmol/L) | 19.0±8.3 | 15.3±6.9 | <0.001 |
| PT (s) | 13.8±0.9 | 13.8±1.0 | 0.939 |
| INR | 1.06±0.07 | 1.06±0.07 | 0.939 |
| ALBI | −2.58±0.37 | −2.69±0.42 | 0.046 |
| PNI | 47.6±6.1 | 48.0±6.5 | 0.637 |
| CRP (mg/L) | 1.8 (0.2–86.2) | 1.3 (0.1–59.5) | 0.102 |
| AFP (ng/mL) | 18.3 (1.5–251,299.0) | 26.4 (1.0–114,971.0) | 0.833 |
| HBV surface antigen (with) | 62 (78.5) | 13 (8.3) | 0.981 |
| Cirrhosis (with) | 52 (65.8) | 109 (69.4) | 0.575 |
| Child-Pugh grade A | 74 (93.7) | 152 (96.8) | 0.258 |
| Maximum tumor diameter (cm) | 4.5 (1.0–15.5) | 3.4 (0.7–15.0) | 0.012 |
| PRS | 0.423 (0.114–1.162) | 0.389 (0.168–1.224) | 0.054 |
| Performance status (0 or 1) | 68 (86.1) | 142 (90.4) | 0.312 |
| ASA physiologic status (1 or 2) | 72 (91.1) | 148 (94.3) | 0.367 |
| SSS | 0.047 (−0.267 to 1.118) | −0.180 (−0.297 to 0.450) | <0.001 |
| Estimated blood loss (mL) | 500 (50–4,000) | 200 (30–2,000) | <0.001 |
| Operation time (min) | 255 (100–650) | 175 (60–400) | <0.001 |
| Laparoscopy | 48 (60.8) | 128 (81.5) | 0.001 |
| CRS | 0.169 (−0.351 to 1.159) | −0.128 (−0.421 to 0.686) | <0.001 |
| BCLC (0/A/B/C) | 12/49/11/7 | 38/94/17/8 | 0.302 |
| TNM (I/II/III) | 57/10/12 | 129/13/15 | 0.206 |
Data are expressed as number, number (%), median (range) or mean ± standard deviation. BMI, body mass index; PT, prothrombin time; INR, international normalized ratio; ALBI, albumin-bilirubin index; PNI, prognostic nutritional index; CRP, C-reactive protein; AFP, alpha-fetoprotein; HBV, hepatitis B virus; PRS, Preoperative Risk Score; SSS, Surgical Stress Score; CRS, Comprehensive Risk Score; ASA, American Society of Anesthesiologists; BCLC, Barcelona Clinic Liver Cancer stage; TNM, tumor node metastasis.
Multivariable analysis and ROC curve of the CRS
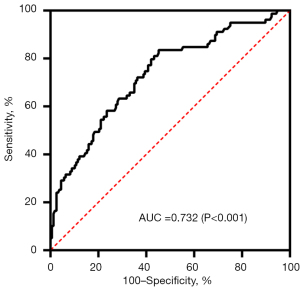
As shown in Table 2, multivariable analysis showed that CRS (OR: 26.556, 95% CI: 7.823–90.147, P<0.001) was independent risk factors for postoperative complications in HCC patients. Furthermore, the ROC curve based on presence of postoperative complications were plotted in order to determine an optimal critical value of the CRS. As shown in Figure 1, the AUC was 0.732. The highest Youden index was 0.383 with the corresponding cutoff value of the CRS as 0.126.
Table 2
| Characteristics | Multivariate | ||
|---|---|---|---|
| OR | 95% CI | P | |
| Total bilirubin (μmol/L) | 1.083 | 1.037–1.130 | <0.001 |
| Maximum tumor diameter (cm) | 1.040 | 0.930–1.163 | 0.493 |
| CRS | 26.556 | 7.823–90.147 | <0.001 |
CI, confidence interval; CRS, Comprehensive Risk Score; OR, odds ratio.
Comparison between the high and low CRS groups
According to the critical value of CRS calculated previously (0.126), HCC patients were further divided into the low CRS group and the high CRS group. Eight-three (35.2%) patients were included in high CRS group while 153 (64.8%) were in low CRS group.
Table 3 shows demographic characteristics between these two groups. Several parameters of E-PASS were statistically different between two groups, including age (P<0.001), diabetes mellitus (P<0.001), PS (P<0.001), ASA (P<0.001), blood loss (P<0.001), operation time (P<0.001) and extent of skin incision (P<0.001). High CRS group had higher PRS [0.454 (0.216–1.224) vs. 0.289 (0.144–0.670), P<0.001] and SSS [0.204 (−0.267 to 1.118) vs. −0.182 (−0.297 to 0.292), P<0.001]. In addition, patients in high CRS group also had lower hemoglobin (P<0.001), albumin (P=0.009) and larger maximum tumor diameter (P<0.001).
Table 3
| Characteristic | CRS <0.126 (n=153) | CRS ≥0.126 (n=83) | P |
|---|---|---|---|
| Age (years) | 57.3±10.8 | 64.2±10.1 | <0.001 |
| Gender (male/female) | 129/24 | 70/13 | 0.096 |
| Weight (kg) | 65.1±10.4 | 63.4±10.5 | 0.259 |
| BMI (kg/m2) | 23.2±3.0 | 23±3.1 | 0.590 |
| Hypertension (with) | 47 (30.7) | 36 (43.4) | 0.052 |
| Diabetes mellitus (with) | 18 (11.8) | 21 (25.3) | 0.008 |
| Hemoglobin (g/L) | 142.6±15.5 | 134.6±17.8 | <0.001 |
| Lymphocyte count (×109/L) | 1.5±0.7 | 1.4±0.6 | 0.103 |
| Neutrophils count (×109/L) | 3.3±1.3 | 3.3±1.5 | 0.891 |
| Platelet (×109/L) | 148.3±55.7 | 145.0±67.2 | 0.701 |
| Albumin (g/L) | 41.0±4.8 | 39.3±4.7 | 0.009 |
| Total bilirubin (μmol/L) | 16.7±7.4 | 16.2±7.9 | 0.634 |
| PT (s) | 13.8±1.0 | 13.8±0.9 | 0.604 |
| ALBI | −2.7±0.4 | −2.6±0.4 | 0.014 |
| PNI | 48.7±6.4 | 46.3±6.0 | 0.004 |
| CRP (mg/L) | 1.2 (0.1–46.6) | 2.2 (0.1–86.2) | <0.001 |
| AFP (ng/mL) | 27.5 (1.0–114,971.0) | 16.2 (1.2–251,299.0) | 0.581 |
| HBV surface antigen (with) | 127 (83.0) | 59 (71.1) | 0.032 |
| Cirrhosis (with) | 107 (69.9) | 54 (65.1) | 0.443 |
| Child-Pugh grade A | 148 (96.7) | 78 (94.0) | 0.328 |
| Maximum tumor diameter (cm) | 3.2 (0.7–15.0) | 5.4 (1.0–15.5) | <0.001 |
| PRS | 0.289 (0.144–0.670) | 0.454 (0.216–1.224) | <0.001 |
| Performance status (0 or 1) | 151 (98.7) | 59 (71.1) | <0.001 |
| ASA physiologic status (1 or 2) | 150 (98.0) | 70 (84.3) | <0.001 |
| SSS | −0.182 (−0.297 to 0.292) | 0.204 (−0.267 to 1.118) | <0.001 |
| Estimated blood loss (mL) | 200 (30–1,500) | 500 (50–4,000) | <0.001 |
| Operation time (min) | 180 (60–400) | 250 (100–650) | <0.001 |
| Laparoscopy | 145 (94.8) | 31 (37.3) | <0.001 |
| CRS | −0.154 (−0.421 to 0.124) | 0.301 (0.129 to 1.159) | <0.001 |
| BCLC (0/A/B/C) | 38/89/18/8 | 12/54/10/7 | 0.258 |
| TNM (I/II/III) | 124/17/12 | 62/6/15 | 0.049 |
Data are expressed as number, number (%), median (range) or mean ± standard deviation. BMI, body mass index; PT, prothrombin time; ALBI, albumin-bilirubin index; PNI, prognostic nutritional index; CRP, C-reactive protein; AFP, alpha-fetoprotein; HBV, hepatitis B virus; PRS, Preoperative Risk Score; SSS, Surgical Stress Score; CRS, Comprehensive Risk Score; ASA, American Society of Anesthesiologists; BCLC, Barcelona Clinic Liver Cancer stage; TNM, tumor node metastasis.
As shown in Table 4, 46 (55.4%) patients in high CRS group suffered from different degrees of postoperative complications, which was significantly higher than that in low CRS group [33 (21.6%), P<0.001]. As to mild complications (Clavien-Dindo Grade II), the incidence was 55.4% in the high CRS group and 15.0% in the low CRS group (P<0.001), including postoperative blood transfusion [26 (31.3%) vs. 12 (7.8%), P<0.001], high fever after surgery (>38.5 ℃) [13 (15.7%) vs. 6 (3.9%), P<0.001], deep venous thrombosis or pulmonary embolus [11 (13.3%) vs. 2 (1.3%), P<0.001] and superficial infections [4 (4.8%) vs. 0, P=0.015]. However, there is no statistical difference in the incidence of atrial fibrillation (P=0.524). In major complications (Clavien-Dindo grade III to grade V), the incidence was higher in high CRS group than another group [28 (33.7%) vs. 13 (8.5%), P<0.001)], including pneumonia [6 (7.2%) vs. 2 (1.3%), P<0.024], intra-abdominal infection [7 (8.4%) vs. 0, P=0.001], puncture in thoracic or abdominal effusion [20 (24.1%) vs. 9 (5.9%), P<0.001], postoperative hemorrhage [6 (7.2%) vs. 1 (0.7%), P=0.008], Single or multiple organs dysfunction [5 (6.0%) vs. 1 (0.7%), P=0.021] and dead case [6 (7.2%) vs. 0, P=0.002]. Biliary fistula or shock showed no difference statistically (P=0.123, P=0.348 respectively).
Table 4
| Characteristic | CRS ≥0.126 (n=83), n (%) | CRS <0.126 (n=153), n (%) | P |
|---|---|---|---|
| Overall complications | 46 (55.4) | 33 (21.6) | <0.001 |
| Mild complications (grade II) | 46 (55.4) | 23 (15.0) | <0.001 |
| Postoperative blood transfusion | 26 (31.3) | 12 (7.8) | <0.001 |
| Postoperative fever >38.5 ℃ | 13 (15.7) | 6 (3.9) | <0.001 |
| DVT or pulmonary embolus | 11 (13.3) | 2 (1.3) | <0.001 |
| Atrial fibrillation | 5 (6.0) | 6 (3.9) | 0.524 |
| Superficial infections | 4 (4.8) | 0 (0) | 0.015 |
| Major complications (grade III to grade V) | 28 (33.7) | 13 (8.5) | <0.001 |
| Pneumonia | 6 (7.2) | 2 (1.3) | 0.024 |
| Intra-abdominal infection | 7 (8.4) | 0 (0) | 0.001 |
| Biliary fistula | 2 (2.4) | 0 (0) | 0.123 |
| Puncture in thoracic or abdominal effusion | 20 (24.1) | 9 (5.9) | <0.001 |
| Postoperative hemorrhage | 6 (7.2) | 1 (0.7) | 0.008 |
| Single organ dysfunction | 5 (6.0) | 1 (0.7) | 0.021 |
| Multiple organs dysfunction | 5 (6.0) | 1 (0.7) | 0.021 |
| Shock | 3 (3.6) | 2 (1.3) | 0.348 |
| Dead case | 6 (7.2) | 0 (0) | 0.002 |
CRS, Comprehensive Risk Score; DVT, deep venous thrombosis.
Analyses of possible factors in relation to RFS and OS
As presented in Table 5, in univariate analysis of RFS, only maximum tumor diameter was the predictor (HR: 1.090, 95% CI: 1.013–1.172, P=0.021). In univariate analysis of OS, nine factors including CRS were considered significant. Before multivariate analyses, we excluded two variables (albumin and SSS). In the end, CRS (P=0.023) and maximum tumor diameter (P<0.001) were significant independent predictors of OS. ALL cases were separated into two groups according to the cutoff value of CRS (0.126).
Table 5
| Variables | RFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate, HR (95% CI) | P | Univariate | Multivariate | |||||
| HR (95% CI) | P | HR (95% CI) | P | |||||
| Age <65 years | 1.061 (0.669–1.683) | 0.801 | 1.089 (0.571–2.077) | 0.796 | – | – | ||
| Gender (female) | 0.744 (0.383–1.444) | 0.382 | 1.183 (0.546–2.564) | 0.671 | – | – | ||
| BMI (<25 kg/m²) | 0.788 (0.490–1.266) | 0.324 | 1.104 (0.541–2.251) | 0.787 | – | – | ||
| HBsAg (positive) | 1.462 (0.820–2.605) | 0.198 | 2.187 (0.858–5.575) | 0.101 | – | – | ||
| Cirrhosis (positive) | 1.309 (0.800–2.142) | 0.283 | 1.817 (0.867–3.808) | 0.114 | – | – | ||
| Albumin (<35 g/L) | 1.635 (0.917–2.917) | 0.096 | 2.619 (1.312–5.229) | 0.006 | – | – | ||
| Total bilirubin (<17.1 μmol/L) | 1.011 (0.645–1.585) | 0.961 | 1.429 (0.740–2.760) | 0.288 | – | – | ||
| AFP (<400 μg/L) | 0.805 (0.465–1.394) | 0.440 | 0.567 (0.284–1.132) | 0.108 | – | – | ||
| ALBI | 1.376 (0.794–2.383) | 0.255 | 2.517 (1.213–5.226) | 0.013 | 0.917 (0.186–4.515) | 0.915 | ||
| PNI | 0.976 (0.942–1.012) | 0.185 | 0.935 (0.890–0.982) | 0.008 | 0.955 (0.858–1.063) | 0.401 | ||
| PRS | 0.989 (0.276–3.537) | 0.986 | 1.493 (0.266–8.394) | 0.649 | – | – | ||
| SSS | 1.329 (0.510–3.467) | 0.561 | 10.066 (3.571–28.372) | <0.001 | – | – | ||
| CRS | 1.189 (0.548–2.579) | 0.661 | 5.725 (2.288–14.320) | <0.001 | 3.735 (1.200–11.631) | 0.023 | ||
| Maximum tumor diameter (cm) | 1.090 (1.013–1.172) | 0.021 | 1.263 (1.170–1.363) | <0.001 | 1.179 (1.078–1.289) | <0.001 | ||
| Tumor number (≥2) | 1.283 (0.678–2.427) | 0.443 | 1.273 (0.535–3.027) | 0.585 | – | – | ||
| Tumor thrombosis (positive) | 1.096 (0.346–3.475) | 0.876 | 2.724 (0.970–7.650) | 0.057 | – | – | ||
| Micro thrombosis | 1.231 (0.634–2.390) | 0.539 | 2.283 (1.089–4.785) | 0.029 | 1.999 (0.771–5.183) | 0.154 | ||
| Postoperative TACE (positive) | 0.649 (0.417–1.010) | 0.055 | 1.487 (0.758–2.915) | 0.248 | – | – | ||
| TNM (I) | 0.669 (0.406–1.102) | 0.114 | 0.501 (0.262–0.956) | 0.036 | 1.341 (0.419–4.294) | 0.621 | ||
| BCLC (0 & A) | 0.796 (0.460–1.379) | 0.416 | 0.458 (0.237–0.885) | 0.020 | 0.764 (0.263–2.217) | 0.620 | ||
HCC, hepatocellular carcinoma; BMI, body mass index; RFS, recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; HBsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein; ALBI, albumin bilirubin index; PNI, prognostic nutritional index; PRS, Preoperative Risk Score; SSS, Surgical Stress Score; CRS, Comprehensive Risk Score; BCLC, Barcelona Clinic Liver Cancer stage; TNM, tumor node metastasis; TACE, transarterial chemoembolization.
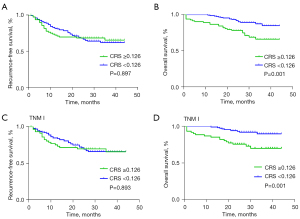
In RFS, there was no significant difference between two groups (P=0.897, Figure 2A). In OS, the 3-year OS of CRS high group (CRS ≥0.126) was 66.2% and CRS low group (CRS <0.126) was 84.8% (P=0.001, Figure 2B). In patients with TNM I, RFS showed no difference between CRS high group and low group (P=0.893, Figure 2C). Similarly, patients in staging TNM I with high CRS may had a worse long-term prognosis (P=0.001, Figure 2D). These results showed that the higher CRS was associated with worse OS.
Discussion
In this study, we assessed the predictive power of the E-PASS models in postoperative complications of HCC patients firstly. For these patients, our results revealed that the CRS of E-PASS system was associated with the occurrence of postoperative complications independently, indicating that a higher CRS was associated with a higher risk. HCC accounts for the vast majority of the liver malignancies. Surgical resection and liver transplantation have been the basic curative therapy in early-stage cases, with a 5-year survival up to 70–80% (5,29,30). Therefore, it is necessary to evaluate the risks and benefits of surgery for HCC patients.
E-PASS system which proposed by Haga et al. took preoperative factors (PRS) and surgical factors (SSS) into account, which has been proven to predict incidence of morbidity and mortality after digestive surgeries (11,12,31). In this system, PRS acts as a comprehensive assessment of the preoperative physiological state of patients. In our study, most of the cases were in low score of PS index or ASA physiologic status and seldom had severe heart or pulmonary disease. Therefore, physiologic risk of cases in our study were low, and PRS showed no difference between our two groups. Nanashima et al. (19) analyzed elderly HCC patients underwent operations, and PRS significantly differed between subgroups which divided by age. It was showed in previous study that operative risk is higher in elderly patients as deterioration of liver functions with age (32). In addition, elderly patients were usually accompanied by other diseases and higher risk of anesthesia (33). The weight of PRS in CRS cannot be ignored. Similar to other previous literature (13,14,34,35), the SSS which reflects the surgical stress had significant predictive ability between two groups in our study. Both operation time and blood loss were higher in groups suffered postoperative complication. Intraoperative blood loss has been reported as a postoperative predictor of liver failure and postoperative morbidity (36,37). Various host responses induced by surgical intervention were activated to maintain individual homeostasis. The balanced host defense mechanism may be destroyed if the surgical stress exceeds patient’s reserve capacity. Considering that CRS mainly reflect the patient’s preoperative reserve capacities and surgical pressure comprehensively, it may be more accurate in predicting the occurrence of postoperative complications theoretically, which requires a larger sample of research to confirm. Preoperatively, by estimating the patient’s SSS through previous similar operations and evaluating the PRS, the surgeon can get an approximate CRS, which could predict postoperative morbidity rates of surgical procedures. If an estimated CRS exceeds 1.0, the surgeon should consider revising the surgical plan to reduce the CRS and improve postoperative outcome (31). Haga et al. (11) indicated that the SSS potentially has a better correlation with postoperative complications than the PRS in younger patients, which means that SSS may bears more weight than the PRS in E-PASS system. Undoubtedly, surgeons should try their best to reduce the operation time and blood loss by optimizing operation process to minimize surgical stress.
Both tumor diameter and ALBI score were significant different between two groups. Larger tumors may be related to larger wounds and more bleeding, which could get a higher SSS and CRS. Comparing with Child-Pugh score, ALBI score was validated to stratify the risk of HCC patients undergoing liver resection more accurately (28,38). This score, comprised of serum bilirubin and albumin, acts as a liver function measuring model. E-PASS system does not include evaluation of liver function directly, thus restricting the applications of this system in liver operation. There is, however, up to 90% patients occurring HCC were in a background of cirrhosis (6,39). It was reported that liver resection in HCC patients of Child-Pugh B grade or accompanied by portal vein hypertension may resulted in a 5-year survival less than 50% and a perioperative mortality of 4% (40). Liver function evaluation preoperatively matters for patients undergoing liver resection. Except for ALBI score and Child-Pugh score, indocyanine green clearance has been used conventionally to assess liver function prior to resection (29). Using imaging technology as assessment tools for liver function have been reported, but the results are still not convincing (41). E-PASS system has been verified as an effective model for predicting postoperative morbidity and mortality. If E-PASS system could be combined with other indicators of liver function, its application value in the field of liver surgery will increase.
Furthermore, we plotted a ROC curve to decide an optimal cutoff value of the CRS for predicting postoperative complication. According to the curve, we selected 0.126 as the cutoff value with the sensitivity =0.835, the specificity= 0.548 and AUC =0.732. The cut-off value of CRS differs in different literatures due to differences in research topics and methods. Previous study has shown that CRS >0.5 was associated with a higher incidence of poor postoperative events (12). There are several possible reasons to account for low CRS levels in our research. First of all, as we described before, PRS were low because of our cases were in a relatively balanced physiological state. Secondly, the use of laparoscopy continues to increase and 73.9% patients underwent laparoscopic surgery. According to the algorithm of the E-PASS system, laparoscopic surgery is assigned 0 points while laparotomy is 1. Difference of extent of skin incision ultimately accounted for a gap of 0.344 points in the CRS.
Referring to our CRS cutoff valve, the cases were divided into high-CRS group and low-CRS group. The analytical results indicated that patients with low CRS were less likely to develop postoperative complications after surgery statistically, including mild and major complications. But our results found no significant difference in postoperative atrial fibrillation and shock. Usually, mild complications are not fatal, but major complications are probably associated with poor perioperative prognosis in patients. All deaths were observed to have multiple serious complications, such as pneumonia, organ dysfunction or shock.
We also supposed CRS is related to RFS or OS of HCC patients (Table 5). In univariate analyze of survive, we found that high PNI may be a protective factor for long-term survival. Low PNI as reported previously was an independent poor prognostic factor in HCC patients received hepatectomy (42). PNI model contains two parameters: peripheral lymphocyte count and serum albumin, which reflects individual’s nutritional and immunological status. Low PNI indicates poor nutritional status or immune malfunction, which may be related with higher tumor burden and promotes cancer progression (43). The result also showed that CRS and maximum tumor diameter were independent prognostic factors of OS in our study. Tumor staging system usually regard tumor diameter as an important parameter. Patients in early stage (TNM = I or BCLC = 0 or A) showed better prognosis in univariate analysis. The relationship between CRS and long-term prognosis is uncertain. In fact, except for patients who died due to severe postoperative complications, the majority of deaths in our study were accompanied by tumor recurrence or metastasis. Most previous studies focused on mortality and morbidity in perioperative period (12,13,15,18,31). Although our research found that high CRS was correlated with poor long-term prognosis. Considering that parameters contained in E-PASS model seem not to be related to recurrence and metastasis of tumor directly (11), more researches will be needed to give a comprehensive explanation. However, our results may be a reference for surgeons in treatment decision when facing patients with poor physiological conditions or estimated long operation time.
There were some limitations in our study. Firstly, the number of patients was limited, so the cutoff value of CRS in HCC patients remains correction. Secondly, the fellow-up time was not long enough. Thirdly, our study did not take postoperative antiviral-therapy or immunotherapy, etc. into consideration.
Conclusions
E-PASS is an effective predictive system for evaluating the risk of postoperative complications of liver resection in HCC patients. This system has many advantages, such as easily accessible data and simple evaluation steps, as well as incorporation of preoperative measurements with intraoperative measurements. Preoperative prediction of CRS can give surgeons guidance. Patients with higher CRS should be given effective preoperative management to reduce postoperative complications and improve prognosis. E-PASS system may be a predictor of the long-term prognosis of HCC patients undergoing resection. In addition, this system requires further evaluation and correction to fit different kinds of surgeries.
Acknowledgments
We are grateful to Yiyin Zhang for her language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-352/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-352/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-352/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-352/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Huitzil-Melendez FD, Capanu M, O'Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 2010;28:2889-95. [Crossref] [PubMed]
- Llovet JM, Villanueva A, Marrero JA, et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology 2021;73:158-91. [Crossref] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Garrido A, Djouder N. Cirrhosis: A Questioned Risk Factor for Hepatocellular Carcinoma. Trends Cancer 2021;7:29-36. [Crossref] [PubMed]
- Berzigotti A, Reig M, Abraldes JG, et al. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 2015;61:526-36. [Crossref] [PubMed]
- Cescon M, Vetrone G, Grazi GL, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg 2009;249:995-1002. [Crossref] [PubMed]
- Kobayashi K, Kawaguchi Y, Schneider M, et al. Probability of Postoperative Complication after Liver Resection: Stratification of Patient Factors,Operative Complexity, and Use of Enhanced Recovery after Surgery. J Am Coll Surg 2021;233:357-368.e2. [Crossref] [PubMed]
- Mise Y, Hasegawa K, Satou S, et al. How Has Virtual Hepatectomy Changed the Practice of Liver Surgery?: Experience of 1194 Virtual Hepatectomy Before Liver Resection and Living Donor Liver Transplantation. Ann Surg 2018;268:127-33. [Crossref] [PubMed]
- Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today 1999;29:219-25. [Crossref] [PubMed]
- Haga Y, Wada Y, Takeuchi H, et al. Estimation of physiologic ability and surgical stress (E-PASS) for a surgical audit in elective digestive surgery. Surgery 2004;135:586-94. [Crossref] [PubMed]
- Oka Y, Nishijima J, Oku K, et al. Usefulness of an estimation of physiologic ability and surgical stress (E-PASS) scoring system to predict the incidence of postoperative complications in gastrointestinal surgery. World J Surg 2005;29:1029-33. [Crossref] [PubMed]
- Hashimoto D, Takamori H, Sakamoto Y, et al. Is an estimation of physiologic ability and surgical stress able to predict operative morbidity after pancreaticoduodenectomy? J Hepatobiliary Pancreat Sci 2010;17:132-8. [Crossref] [PubMed]
- Haga Y, Miyamoto A, Wada Y, et al. Value of E-PASS models for predicting postoperative morbidity and mortality in resection of perihilar cholangiocarcinoma and gallbladder carcinoma. HPB (Oxford) 2016;18:271-8. [Crossref] [PubMed]
- Capussotti L, Muratore A, Amisano M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: analysis of mortality, morbidity and survival--a European single center experience. Eur J Surg Oncol 2005;31:986-93. [Crossref] [PubMed]
- Benzoni E, Molaro R, Cedolini C, et al. Liver resection for HCC: analysis of causes and risk factors linked to postoperative complications. Hepatogastroenterology 2007;54:186-9. [PubMed]
- Banz VM, Studer P, Inderbitzin D, et al. Validation of the estimation of physiologic ability and surgical stress (E-PASS) score in liver surgery. World J Surg 2009;33:1259-65. [Crossref] [PubMed]
- Nanashima A, Abo T, Nonaka T, et al. Prognosis of patients with hepatocellular carcinoma after hepatic resection: are elderly patients suitable for surgery? J Surg Oncol 2011;104:284-91. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Fisher JD. New York Heart Association Classification. Arch Intern Med 1972;129:836. [Crossref] [PubMed]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Owens WD, Felts JA, Spitznagel EL Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology 1978;49:239-43. [Crossref] [PubMed]
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001-5. [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019 Apr;70(4):817. [Crossref]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Haga Y, Ikei S, Wada Y, et al. Evaluation of an Estimation of Physiologic Ability and Surgical Stress (E-PASS) scoring system to predict postoperative risk: a multicenter prospective study. Surg Today 2001;31:569-74. [Crossref] [PubMed]
- Nagata T. Macromolecular synthesis in the livers of aging mice as revealed by electron microscopic radioautography. Prog Histochem Cytochem 2010;45:1-79. [Crossref] [PubMed]
- Levine WC, Mehta V, Landesberg G. Anesthesia for the elderly: selected topics. Curr Opin Anaesthesiol 2006;19:320-4. [Crossref] [PubMed]
- Hashimoto D, Takamori H, Sakamoto Y, et al. Can the physiologic ability and surgical stress (E-PASS) scoring system predict operative morbidity after distal pancreatectomy? Surg Today 2010;40:632-7. [Crossref] [PubMed]
- Zhang A, Liu T, Zheng K, et al. Estimation of physiologic ability and surgical stress (E-PASS) scoring system could provide preoperative advice on whether to undergo laparoscopic surgery for colorectal cancer patients with a high physiological risk. Medicine (Baltimore) 2017;96:e7772. [Crossref] [PubMed]
- Tzeng CW, Cooper AB, Vauthey JN, et al. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford) 2014;16:459-68. [Crossref] [PubMed]
- Prodeau M, Drumez E, Duhamel A, et al. An ordinal model to predict the risk of symptomatic liver failure in patients with cirrhosis undergoing hepatectomy. J Hepatol 2019;71:920-9. [Crossref] [PubMed]
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338-46. [Crossref] [PubMed]
- Bartolomeo N, Trerotoli P, Serio G. Progression of liver cirrhosis to HCC: an application of hidden Markov model. BMC Med Res Methodol 2011;11:38. [Crossref] [PubMed]
- Berardi G, Morise Z, Sposito C, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol 2020;72:75-84. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Shirata C, et al. Assessment of Preoperative Liver Function for Surgical Decision Making in Patients with Hepatocellular Carcinoma. Liver Cancer 2019;8:447-56. [Crossref] [PubMed]
- Fan X, Chen G, Li Y, et al. The Preoperative Prognostic Nutritional Index in Hepatocellular Carcinoma After Curative Hepatectomy: A Retrospective Cohort Study and Meta-Analysis. J Invest Surg 2021;34:826-33. [Crossref] [PubMed]
- Zhang Z, Pereira SL, Luo M, et al. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017;9:829. [Crossref] [PubMed]


