
Male triple negative axillary accessory breast cancer—a case report
Introduction
Breast cancer is the most common malignancy among women worldwide (1). Compare to female breast cancer, the incidence of male breast cancer is low, accounting for less than 1% of all cases of breast cancer (2). Majority of male breast cancer cases are hormone-receptor positive (3,4). It has been reported that only 0.2–1.2% of men have axillary accessory breast (5). Cases of male breast cancer occurring in axillary accessory breast are rare. Here, we report a case of male triple negative axillary accessory breast cancer. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-33/rc).
Case presentation
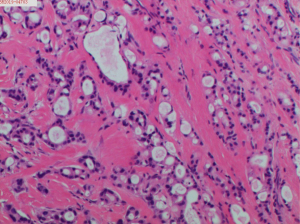
In November 2019, a 67-year-old man presented to our Department of Breast Surgery, Hwa Mei Hospital, University of Chinese Academy of Sciences with one-year history of a right axillary mass, which progressively increased in size. The patient did not report a history of benign breast disease or a history of cancer. He also denied his family history of breast cancer or ovarian cancer. He did not turn to the hospital before and no interventions were taken. Physical examination revealed a 3 cm × 2 cm mass in the right axilla. Ultrasound examination showed a 31 mm × 17 mm mass in the right axillary tail region with some accessory breast tissue around and multiple enlarged lymph nodes (Figures 1,2). No masses were found in both breasts in mammography and ultrasound tests. No obvious abnormality was found in liver ultrasound, Chest computed tomography and tests for tumor makers. On the basis of the aforementioned findings, the patient was diagnosed with axillary accessory breast tumor. Consequently, we performed right axillary accessory tumor resection on November 19, 2019. Intraoperative pathological examination showed invasive cancer, originating from accessory breast firstly considered. We suspected axillary lymph node metastasis and performed right accessory breast resection and right axillary lymph nodes dissection. Postoperative pathological analysis revealed right accessory breast invasive ductal carcinoma with apocrine metaplasia. The tumor size was 3.5 cm × 3.3 cm. In addition, 5 metastatic lymph nodes were seen in 27 axillary lymph nodes. Immunohistochemistry showed ER (−), PR (−), Ki-67 30%, HER2 (2+), GATA-3 (+), GCDFP-15 (+), and AR (+) (Figure 3). FISH test obtained a negative result. The patient was diagnosed with T2N2M0, IIIA stage male triple negative axillary accessory breast cancer. Thus, he was treated with epirubicin and cyclophosphamide (EC) (E: 90 mg/m2, C: 600 mg/m2) every three weeks for 4 cycles, followed by 4 cycles of docetaxel (100 mg/m2) every three weeks. He was subsequently treated with adjuvant radiotherapy after chemotherapy. Irradiation was given to the whole breast, chest, supraclavicular lymph nodes and infraclavicular lymph nodes. The patient received 50 Gy intensity-modulated radiotherapy (IMRT) to the breast and chest 5 days per week for 5 weeks, 16 Gy three-dimensional conformal radiotherapy to the supraclavicular lymph nodes and infraclavicular lymph nodes for 8 days and 34 Gy electron beams as boost for 17 days. The patient adhered to all the adjuvant therapy and adverse events such as alopecia, neutropenia and rash were well tolerated. Until now, no obvious signs of recurrence or metastasis have been observed during regular follow-ups.
All procedures performed in this study were in accordance with the ethical standards of the Hwa Mei Hospital, University of Chinese Academy of Sciences research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
A United States study showed male triple-negative breast cancer accounted for 5.5% of the male breast cancer (6), while Pang reported the incidence rate of accessory breast cancer is 0.3–0.6% of all the breast cancer (7). Given that majority of male axillary accessory breast degenerate, there are few cases of male axillary accessory breast (8). The incidence of male axillary accessory breast cancer is very low. For this reason, no large-scale prospective randomized clinical trials have been conducted to determine effective therapy for this condition (9). Currently, male axillary accessory breast cancer is treated similar to female breast cancer (10).
Sometimes it is difficult to distinguish axillary accessory breast cancer from adnexal and skin appendage neoplasm, especially for triple negative tumors. For this patient, there was a clinical suspicious accessory breast tissue with enlargement of the right axilla by physical examination and ultrasound showing some accessory breast tissue. Immunohistochemistry showed ER (−), PR (−), Ki-67 30%, HER2 (2+), GATA-3 (+), GCDFP-15 (+) with FISH negative. At the same time, we found normal accessory breast tissue next to the accessory breast tumor in Hematoxylin Eosin staining. So, the mass was finally diagnosed axillary accessory breast cancer. Pathological examination confirmed invasive ductal carcinoma with apocrine metaplasia. Less than 90% of the cancer cells showed morphological and immunohistochemical characteristics of apocrine gland cells. Hence, the patient was diagnosed with invasive ductal carcinoma with apocrine metaplasia instead of apocrine carcinoma (11). The cancer was estrogen receptor negative and progesterone receptor negative, possibly due to the apocrine metaplasia, which is rare in male breast cancer.
Operable male axillary accessory breast cancer is comprehensively treated with surgery (10). In general, mastectomy with sentinel lymph nodes biopsy is performed (10,12). For patients suspected with axillary lymph node metastasis, axillary lymph nodes dissection can be performed directly instead of sentinel lymph node biopsy. Compelling evidence indicates that breast conserving surgery is safe and feasible for male breast cancer (13,14). However, in clinical practice, male breast cancer occurs near the nipple in most cases and majority of patients with male breast cancer do not have a strong need undergo a breast-conserving surgery. Most patients with male breast cancer receiving treatment in our department agreed to undergo breast resection. In the present case, with the clinical suspicion of axillary lymph nodes metastasis, the patient underwent accessory breast resection and axillary lymph node dissection.
The application of chemotherapy and radiotherapy in male patients with axillary accessory breast cancer is similar to that of female patients with breast cancer (10,15). The prognosis of patients with male breast cancer is worse compared to female patients with breast cancer (16). In our clinical practice, most male patients with breast cancer are given chemotherapy containing anthracycline or paclitaxel. We often assess the prognosis of patients using oncotype DX and other gene prognostic models to decide the appropriate chemotherapy (17,18). The adjuvant radiotherapy applied to male breast cancer is similar to that used for female patients with breast cancer. Adjuvant radiotherapy is recommended for patients with axillary lymph nodes metastasis or undergoing breast conserving surgery. Postoperative radiotherapy is also recommended for patients with tumors larger than 5 cm. In our case, the patient was diagnosed with T2N2M0, IIIA stage male triple negative axillary accessory breast cancer. Ki-67 was 30%. He received 8 cycles of chemotherapy comprising anthracycline and paclitaxel. We recommended dose-dense chemotherapy for every 2 weeks but the patient refused due to poor tolerance. We adjusted the cycle of chemotherapy to every 3 weeks. With 5 out of 27 lymph nodes metastasis, the patient received adjuvant radiotherapy after chemotherapy. The axilla was not radiated to avoid edema of upper limbs.
Though refusing dose-dense chemotherapy, the patient behaved well with adjuvant chemotherapy and radiotherapy. Adverse events such as alopecia, neutropenia and rash were well tolerated. He recovered well after surgery and adjuvant therapy. With no signs of recurrence or metastasis observed during regular follow-ups, he is satisfied with the therapy he received.
This is a rare case of male triple negative axillary accessory breast cancer. Currently, Male axillary accessory breast cancer is treated similar to female breast cancer with combined therapy including surgery and chemotherapy.
Acknowledgments
The authors thank the members of their department for their assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-33/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-33/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-33/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Hwa Mei Hospital, University of Chinese Academy of Sciences research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Fox S, Speirs V, Shaaban AM. Male breast cancer: an update. Virchows Arch 2022;480:85-93. [Crossref] [PubMed]
- Serdy KM, Leone JP, Dabbs DJ, et al. Male Breast Cancer. Am J Clin Pathol 2017;147:110-9. [PubMed]
- Liu N, Johnson KJ, Ma CX. Male Breast Cancer: An Updated Surveillance, Epidemiology, and End Results Data Analysis. Clin Breast Cancer 2018;18:e997-e1002. [Crossref] [PubMed]
- Bi M, Li D, Su Y, et al. Male axillary accessory breast cancer: A case report. Medicine (Baltimore) 2020;99:e19506. [Crossref] [PubMed]
- Yadav S, Karam D, Bin Riaz I, et al. Male breast cancer in the United States: Treatment patterns and prognostic factors in the 21st century. Cancer 2020;126:26-36. [Crossref] [PubMed]
- Pang L, Cui M, Dai W, et al. Diagnosis and Treatment of Male Accessory Breast Cancer: A Comprehensive Systematic Review. Front Oncol 2021;11:640000. [Crossref] [PubMed]
- Lee SR, Lee SG, Byun GY, et al. Axillary Accessory Breast: Optimal Time for Operation. Aesthetic Plast Surg 2018;42:1231-43. [Crossref] [PubMed]
- Duma N, Hoversten KP, Ruddy KJ. Exclusion of Male Patients in Breast Cancer Clinical Trials. JNCI Cancer Spectr 2018;2:pky018. [Crossref] [PubMed]
- Giordano SH. Breast Cancer in Men. N Engl J Med 2018;378:2311-20. [Crossref] [PubMed]
- D'Arcy C, Quinn CM. Apocrine lesions of the breast: part 2 of a two-part review. Invasive apocrine carcinoma, the molecular apocrine signature and utility of immunohistochemistry in the diagnosis of apocrine lesions of the breast. J Clin Pathol 2019;72:7-11. [Crossref] [PubMed]
- Hassett MJ, Somerfield MR, Baker ER, et al. Management of Male Breast Cancer: ASCO Guideline. J Clin Oncol 2020;38:1849-63. [Crossref] [PubMed]
- Zaenger D, Rabatic BM, Dasher B, et al. Is Breast Conserving Therapy a Safe Modality for Early-Stage Male Breast Cancer? Clin Breast Cancer 2016;16:101-4. [Crossref] [PubMed]
- Bateni SB, Davidson AJ, Arora M, et al. Is Breast-Conserving Therapy Appropriate for Male Breast Cancer Patients? A National Cancer Database Analysis. Ann Surg Oncol 2019;26:2144-53. [Crossref] [PubMed]
- Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol 2018;29:405-17. [Crossref] [PubMed]
- Elimimian EB, Elson L, Li H, et al. Male Breast Cancer: A Comparative Analysis from the National Cancer Database. World J Mens Health 2021;39:506-15. [Crossref] [PubMed]
- Grenader T, Yerushalmi R, Tokar M, et al. The 21-gene recurrence score assay (Oncotype DX™) in estrogen receptor-positive male breast cancer: experience in an Israeli cohort. Oncology 2014;87:1-6. [Crossref] [PubMed]
- Li C, Li X, Li G, et al. Identification of a prognosis-associated signature associated with energy metabolism in triple-negative breast cancer. Oncol Rep 2020;44:819-37. [Crossref] [PubMed]




