Benzene exposure increases the risk of non-Hodgkin’s lymphoma: a systematic review and meta-analysis of observational studies
Introduction
Non-Hodgkin’s lymphoma (NHL), which includes more than 60 subtypes, is a group of malignant hematological tumors originating from lymphoid tissue (1). Due to heterogeneity between the different subtypes, diagnosis and clinical classification of NHL is very challenging, making evaluation of its epidemiological characteristics even more difficult. Although the exact pathogenesis of most NHL subtypes is not clear, many epidemiological studies have shown that the known risk factors of NHL include genetic differences, viral infection [such as human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), human T-lymphotropic virus type I (HTLV-1), hepatitis C virus (HCV), and Kaposi’s sarcoma-associated herpesvirus (KSHV)], autoimmune regulatory factors (use of immunosuppressants, congenital immunodeficiency, and acquired immunodeficiency), lifestyle habits (smoking and alcohol use), and exposure to certain occupational and environmental factors (hair dyes, oil refining workers, etc.) (2,3). Benzene is listed as a carcinogen by the International Agency for Research on Cancer (IARC) because it directly leads to an increased risk of acute myeloid leukemia (4). In addition, benzene has developmental, neurological, and hepatocyte toxicity. However, due to its simple aromatic structure and the high activity of its metabolites, benzene plays a vital role in the chemical manufacturing industry. It is indispensable in producing key chemicals used in the synthesis of plastics, resins, and other fibers (5). In addition, the ubiquitous presence of benzene in the industry makes its exposure inevitable, while its important economic status has made the relationship between disease and benzene exposure controversial. Previous observational studies have been controversial as to whether benzene exposure increases the risk of NHL. For example, Schumacher et al., Collins et al., Rinsky et al. (6-8) believe that benzene exposure does not increase the risk of NHL, while other studies (9,10) believe that benzene exposure is positively related to the risk of NHL. The method of meta-analysis, by systematically searching, evaluating, and merging all relevant literature, can expand the observed sample size, integrate studies in different regions, and make the research results more general. And the meta-analysis reduces the uncertainty in the decision-making of evidence-based medicine caused by the bias between the studies. Therefore, this study aimed to retrieve observational studies that quantitatively evaluated NHL and benzene exposure in order to provide evidence-based medical data for the prevention of NHL. We present the following article in accordance with the MOOSE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1434/rc).
Methods
Literature search
This study used medical subject headings (MeSH) search words in English databases such as PubMed, Embase, Sciencedirect and other Chinese databases such as Wanfang Database and China National Knowledge Infrastructure (CNKI) for literature retrieval. The included articles were published before 2022. The search keywords were: (“benzene” [MeSH Terms] OR “benzidine” [tiab] OR “petrochemical”) AND (“lymphoma, non-Hodgkin” [MeSH Terms] OR “Chronic lymphocytic leukemia”).
Literature screening
The literature inclusion criteria were: (I) peer-reviewed cohort studies and case-control studies that reported the relative risk (RR) or odds ratio (OR) of benzene exposure to the risk of NHL; (II) The study population was benzene exposed population, and the control population was non-benzene exposed population (III) literature that directly provided the mean and standard deviation or 95% confidence interval (CI) of the risk estimate or could be calculated; (IV) bias and confounding were adjusted and controlled by a multifactor model, and the results had good reliability; and (V) studies with benzene exposure duration, cumulative exposure concentration, or maximum exposure concentration as observation factors.
The literature exclusion criteria were: (I) study populations limited to a particular group, such as a specific occupation or patient group; (II) studies that did not distinguish NHL from other tumor disease types in reported risk estimates; (III) overlapping observation populations between studies, studies with high risk of bias, and studies published previously; and (IV) reviews, academic conferences, and case reports. A total of 14 studies were ultimately included for meta-analysis.
Document data sorting and evaluation
Two researchers independently screened and extracted the following data from the included literature: study design type (cohort, case-control), country or region where the study population was located, number of included observers, measurement method and level of exposure, the estimated value of risk [OR, RR or hazard ratio (HR)], and statistical methods for adjusting confounding or bias. NOS was used to evaluate the methodological quality of the included literature. Literature with a score of less than 5 was considered to be at high risk of bias, 5–8 was considered to be at moderate risk of bias, and literature with a score of 8 or more was considered to be at low risk of bias. If there was disagreement between the 2 researchers, discussion was held with a third researcher to reach a consensus (11).
Statistical methods
STATA 17.0 (Texas, USA) was used in this study for statistical analysis of the data. Since the main clinical indicators observed in this study were continuous variables, they are expressed in the form of mean ± standard deviation. A random-effects model can combine interstudy variance into a total variance estimation and CI to avoid a reduction of the CI caused by a fixed-effect model. However, the random-effects model also has the disadvantage of giving a larger proportion of consolidation to studies with a smaller sample size, and thus the combined estimation is too conservative (12-14). Therefore, we integrated all studies using both random-effects and fixed-effects models, and then pooled risk estimates OR from case-control studies and RR from cohort studies separately (subgroup analysis by study type). Heterogeneity between studies was evaluated using Cochran’s Q test (15,16). If there was significant heterogeneity between studies (I2>50%), the random-effects model was used. Otherwise, the fixed-effect model was used. Publication bias was evaluated by funnel plot and Egger’s and Begg’s tests (17). Hypothesis tests in this study were all two-sided tests. P<0.05 was considered statistically significant.
Results
Search results and literature characteristics
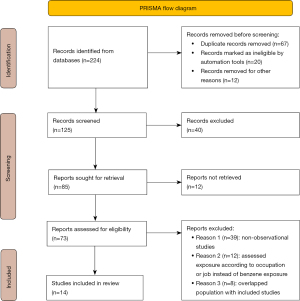
Through a systematic electronic database search, 224 relevant studies were retrieved, of which 14 met the criteria for inclusion in the meta-analysis. A PRISMA flow chart of the literature screening process is shown in Figure 1. The characteristics of the included literature were shown in Table 1. The included literature comprised observational studies, including 9 cohort studies and 5 case-control studies. A total of 1,994 cases of NHL were observed in the 14 studies, which included 492,719 participants. The observation population of 5 studies was considered to have high exposure to benzene (annual average exposure concentration ≥25 ppm or cumulative exposure concentration ≥720 ppm). The other 9 studies involved low to severe exposure levels. In addition, the observation populations came mainly from the United States (n=6), Britain (n=3), China (n=2), Norway (n=1), and Italy (n=2). The NOS scores of the 14 included studies ranged from 5 to 9, of which 4 had low risk of bias and 10 had moderate risk of bias.
Table 1
| Author [year] | RR/OR (95% CI) | Study location | Cases of non-Hodgkin’s lymphoma | Study design | Exposure level | NOS | Exposure level |
|---|---|---|---|---|---|---|---|
| Hayes et al. [1996] (18) | 4.7 (1.2–18.1) | China | 7/19 | Cohort | Higher than 25 ppm/year for average intensity | 7 | High |
| Stenehjem et al. [2015] (19) | 1.55 (0.83–2.88) | Norway | 20/85 | Cohort | 0.013–0.04 ppm for average intensity | 8 | Low to moderate |
| Sorahan et al. [2005] (20) | 1.0 (0.64–1.49) | UK | 24/75 | Cohort | Ever | 6 | Low to moderate |
| Collins et al. [2015] (7) | 0.53 (0.12–1.69) | Michigan, USA | 3/15 | Cohort | Cumulative ≥25 ppm per year | 7 | Low to moderate |
| Bassig et al. [2015] (9) | 2.04 (1.08–3.86) | Shanghai, China | 12/102 | Cohort | Cumulative >102.4 mg/m3 per year (10-year lag) | 8 | High |
| Cartwright et al. [1988] (21) | 1.2 (0.9–1.5) | Yorkshire, UK | 103/153 | Case-control | Ever | 6 | Low to moderate |
| Franceschi et al. [1989] (22) | 1.14 (0.57–2.28) | Italy | 15/28 | Case-control | Ever | 8 | Low to moderate |
| Blair et al. [1993] (23) | 1.5 (0.7–3.1) | Iowa, Minnesota, USA | 12/622 | Case-control | High intensity | 6 | High |
| Ott et al. [1989] (24) | 1.0 (0.83–1.33) | UK | 5/29 | Cohort | Low intensity | 7 | Low to moderate |
| Schumacher [1988] (6) | 0.77 (0.56–1.07) | North Carolina, USA | 56/522 | Case-control | Ever | 7 | Low to moderate |
| Wong [1987] (25) | 4.12 (1.11–10.55) | USA | 4/15 | Cohort | Cumulative ≥720 ppm (adjusted) | 8 | High |
| Rinsky et al. [2002] (8) | 0.96 (0.31–2.25) | Ohio, USA | 5/16 | Cohort | Ever | 5 | Low to moderate |
| Fritschi et al. [2005] (26) | 1.09 (0.75–1.59) | Italy | 347/744 | Case-control | Ever | 7 | Low to moderate |
| Switchenko et al. [2016] (10) | 1.56 (1.44–1.68) | USA | 9/257 | Cohort | Ever | 6 | High |
RR, relative risk; OR, odds ratio; CI, confidence interval; NOS, Newcastle-Ottawa Scale.
Combined effect of benzene exposure on the risk of NHL
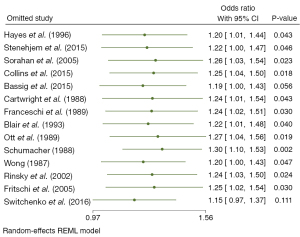
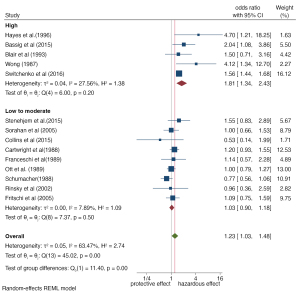
All 14 studies quantified the increased risk of observed outcomes in people exposed to benzene relative to people without benzene exposure, using NHL as the endpoint. The risk estimates in 14 studies were meta-analyzed using the random-effects model and fixed-effect model. Both statistical models showed that benzene exposure increased the risk of NHL. As shown in Figures 2,3, the random-effects model demonstrated that the risk of NHL in the exposed population was 1.23 times higher than that in the nonexposed population (OR =1.23, 95% CI: 1.01, 1.51, P=0.03). The combined risk estimate of the random-effects model was higher than that of the fixed-effect model, which found that the risk of lymphoma in the exposed population increased by only 41% compared with the nonexposed population (RR =1.41, 95% CI: 1.31, 1.52, P=0.00). Both models showed some heterogeneity between the included studies, with I2=63.47% in the random-effects model and I2=71.12% in the fixed-effect model, although the difference was not statistically significant. As a result, we conducted subgroup analysis to explore the possible sources of heterogeneity to provide further basis for the inclusion of more homogeneous literature in the future.
Subgroup analysis and sensitivity analysis
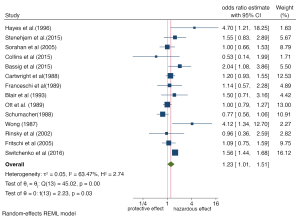
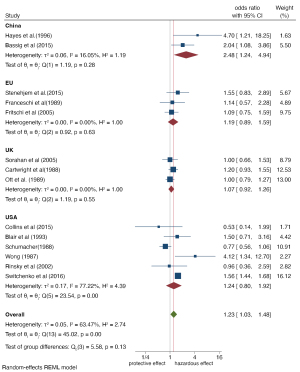
Subgroup analysis was carried out for different study regions, study design types, and exposure levels of benzene. The forest maps are shown in Figures 4-6. Overall, subgroup analysis showed that the exposure level of benzene (Q=11.40, P≤0.001) and the region of the study population (Q=5.58, P=0.13) were the main sources of heterogeneity, while no significant difference was found for study design type (Q=2.28, P=0.13). The Meta-OR of the case-control study subgroup: 1.06 (95% CI: 0.84, 1.32), and the Meta-RR of the cohort study subgroup: 1.39 (95% CI: 1.06, 1.81). Subgroup analysis of exposure levels showed that the risk of NHL increased by 0.81 times in people with high benzene exposure compared with those without exposure (OR =1.81, 95% CI: 1.34, 2.43), and the heterogeneity was very low (I2=27.56%). The risk estimates of the low and medium exposure groups were closer to the total combined estimates (OR =1.03, 95%: 0.90, 1.18, I2=7.89%). Risk estimates from studies based on the Chinese population (OR =2.48, 95% CI: 1.24, 4.94) were greater than those based on the populations of Europe (OR =1.19, 95% CI: 0.89, 1.59), the United Kingdom (OR =1.07, 95% CI: 0.92, 1.26), and the United States (OR =1.24, 95% CI: 0.80, 1.92). Regional differences may be due to the stricter control of occupational benzene exposure in developed countries (the United States has an average of 1 ppm every 8 hours, while China had 12 ppm in 1979–2002 and has had 2 ppm since 2002) (27).
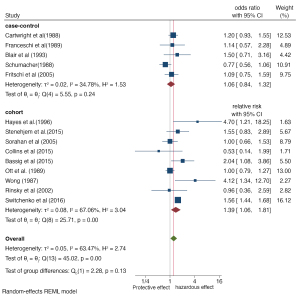
To evaluate the impact of each included study on meta-analysis, we conducted sensitivity analysis and excluded 12 studies one at a time, with the results remaining consistent. As shown in Figure 7, the overall range of OR values of sensitivity analysis was 1.15–1.30. The upper and lower limits were the risk estimates after excluding the studies of Switchenko et al. (10) and Schumacher et al. (6) Overall, robust sensitivity analysis showed that our results were reliable.
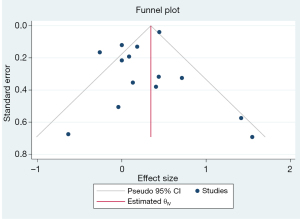
Publication bias
The funnel chart (Figure 8) describing the overall symmetrical distribution of each study was in the shape of an inverted funnel. Within the 95% CI, Egger’s test (t=1.55, P=0.121) illustrated no significant publication bias in this meta-analysis.
Discussion
This meta-analysis provided new evidence-based medical data that benzene exposure increases the risk of NHL to some extent. Although there was no significant difference between the random-effects model and the fixed-effect model, the results of subgroup analysis and sensitivity analysis showed that there was still an epidemiological causal.
This study included 492,719 cases of observation, among which there were 1,994 cases of NHL. The incidence of NHL in this group was significantly higher than that in the general population reported by the literature. In a previous study, the incidence rate of NHL was 7.8 males/10,000 and 5.6 females/10,000 (28). The results of this study demonstrated a causal relationship between benzene exposure and the increased risk of NHL and was in line with Bradford Hill criteria for establishing causal inference, to a certain extent (29). First, the fixed-effect model of meta-analysis showed that benzene exposure increased the risk of NHL by 10% (OR =1.41, 95% CI: 1.31, 1.52, P=0.00), while the random-effects model, with lower heterogeneity, revealed a value of 23% (OR =1.23, 95% CI: 1.01, 1.51, P=0.031). Second, correlation results showed consistency among cohort studies, case-control studies, different exposure levels, and different study regions. Third, in subgroup analysis, we found that the risk of NHL in people exposed to high-dose benzene was significantly higher than that in people exposed to low and medium-dose benzene (OR =1.81, 95% CI: 1.34, 2.43). Most of the included studies (n=9) were prospective cohort studies and showed that benzene exposure was related to the risk of NHL over time, and the results were statistically significant. A previous animal experiment also showed that benzene exposure is associated with the risk of NHL (30). Fourth, the causal relationship between exposure and disease was exclusive and did not include other malignant hematological tumors such as Hodgkin’s lymphoma.
The pathogenic mechanism of NHL is mainly related to immunosuppression and the presence of autoimmune disease. Many epidemiological and experimental studies have shown that chronic inhalation of benzene damages the human immune system by reducing the level of circulating B lymphocytes, blood immunoglobulin, T lymphocytes, and interleukin-2 (3,6,31). In addition, benzene mediates oxidative damage of related DNA and chromosomes by inducing oxidative responses such as reducing the level of serum glutathione, increasing lipid peroxide, reactive oxygen species, and oxidative protein damage, and decreasing the ability of antioxidants (32). In addition, studies have shown that benzene is genotoxic and can induce DNA damage and chromosome changes. Specific cytogenetic changes, including aneuploidy, chromosome translocation, and various other structural chromosome changes, have also been observed in people exposed to benzene (33-35).
All meta-analyses inevitably have limitations. This study did not review the incidence risk of benzene exposure in various subtypes of NHL. Further, this study did not include all literature that classified NHL. However, the diagnostic and typing criteria of NHL were revised in 2008. More importantly, bias in observational studies may mask causality between disease subtypes and benzene exposure. Most of the observational studies included in this study were published between 1987 and 2015. The World Health Organization revised the definition of lymphoma classification in 2008 and further divided lymphoma into T-cell and B-cell lymphoma by integrating clinical, molecular, biological, and morphological evidence in 2014. These changes in the diagnostic criteria of NHL may have led to the misclassification of nondifferential observations, thus affecting the results of our meta-analysis. In addition, given the variability of chronic lymphoid leukemia, which is highly similar to lymphoma in clinical diagnosis, observational studies may underestimate the real risk estimate by minimizing the actual number of patients with NHL. There were 4 case-control studies included in this study. In such studies, exposure estimates are usually based on participant recall, with recall bias possibly leading to misclassification of exposure. However, the combined value of risk in subgroup analysis of cohort studies and case-control studies was comparable, which to some extent showed that study design was not a primary source of offset.
In conclusion, the results of our study suggested that benzene exposure increased the risk of NHL. The results were statistically and biologically feasible and met most of the Bradford Hill criteria for causal inference.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1434/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1434/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nogai H, Dörken B, Lenz G. Pathogenesis of non-Hodgkin's lymphoma. J Clin Oncol 2011;29:1803-11. [Crossref] [PubMed]
- Farris GM, Robinson SN, Wong BA, et al. Effects of benzene on splenic, thymic, and femoral lymphocytes in mice. Toxicology 1997;118:137-48. [Crossref] [PubMed]
- Kirkeleit J, Ulvestad E, Riise T, et al. Acute suppression of serum IgM and IgA in tank workers exposed to benzene. Scand J Immunol 2006;64:690-8. [Crossref] [PubMed]
- Loomis D, Guyton KZ, Grosse Y, et al. Carcinogenicity of benzene. Lancet Oncol 2017;18:1574-5. [Crossref] [PubMed]
- Poça KSD, Giardini I, Silva PVB, et al. Gasoline-station workers in Brazil: Benzene exposure; Genotoxic and immunotoxic effects. Mutat Res Genet Toxicol Environ Mutagen 2021;865:503322. [Crossref] [PubMed]
- Schumacher MC, Delzell E. A death-certificate case-control study of non-Hodgkin's lymphoma and occupation in men in North Carolina. Am J Ind Med 1988;13:317-30. [Crossref] [PubMed]
- Collins JJ, Anteau SE, Swaen GM, et al. Lymphatic and hematopoietic cancers among benzene-exposed workers. J Occup Environ Med 2015;57:159-63. [Crossref] [PubMed]
- Rinsky RA, Hornung RW, Silver SR, et al. Benzene exposure and hematopoietic mortality: A long-term epidemiologic risk assessment. Am J Ind Med 2002;42:474-80. [Crossref] [PubMed]
- Bassig BA, Friesen MC, Vermeulen R, et al. Occupational Exposure to Benzene and Non-Hodgkin Lymphoma in a Population-Based Cohort: The Shanghai Women's Health Study. Environ Health Perspect 2015;123:971-7. [Crossref] [PubMed]
- Switchenko JM, Bulka C, Ward K, et al. Resolving uncertainty in the spatial relationships between passive benzene exposure and risk of non-Hodgkin lymphoma. Cancer Epidemiol 2016;41:139-51. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Weed DL. Interpreting epidemiological evidence: how meta-analysis and causal inference methods are related. Int J Epidemiol 2000;29:387-90. [Crossref] [PubMed]
- Stare J, Maucort-Boulch D. Odds ratio, hazard ratio and relative risk. Metodoloski zvezki 2016;13:59-67.
- Etikan I, Alkassim R, Abubakar S. Meta-analysis of using both cohort and case control study. Biom Biostat Int J 2016;3:96-8. [Crossref]
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193-206. [Crossref] [PubMed]
- Kulinskaya E, Dollinger MB. An accurate test for homogeneity of odds ratios based on Cochran’s Q-statistic. BMC Med Res Methodol 2015;15:49. [Crossref] [PubMed]
- Rothstein HR, Sutton AJ, Borenstein M. editors. Publication bias in meta-analysis: Prevention, assessment and adjustments. John Wiley & Sons, 2006.
- Hayes RB, Yin SN, Dosemeci M, et al. Mortality among benzene-exposed workers in China. Environ Health Perspect 1996;104:1349-52. [PubMed]
- Stenehjem JS, Kjærheim K, Bråtveit M, et al. Benzene exposure and risk of lymphohaematopoietic cancers in 25 000 offshore oil industry workers. Br J Cancer 2015;112:1603-12. [Crossref] [PubMed]
- Sorahan T, Kinlen LJ, Doll R. Cancer risks in a historical UK cohort of benzene exposed workers. Occup Environ Med 2005;62:231-6. [Crossref] [PubMed]
- Cartwright RA, McKinney PA, O'Brien C, et al. Non-Hodgkin's lymphoma: case control epidemiological study in Yorkshire. Leuk Res 1988;12:81-8. [Crossref] [PubMed]
- Franceschi S, Serraino D, Bidoli E, et al. The epidemiology of non-Hodgkin's lymphoma in the north-east of Italy: a hospital-based case-control study. Leuk Res 1989;13:465-72. [Crossref] [PubMed]
- Blair A, Linos A, Stewart PA, et al. Evaluation of risks for non-Hodgkin's lymphoma by occupation and industry exposures from a case-control study. Am J Ind Med 1993;23:301-12. [Crossref] [PubMed]
- Ott MG, Teta MJ, Greenberg HL. Lymphatic and hematopoietic tissue cancer in a chemical manufacturing environment. Am J Ind Med 1989;16:631-43. [Crossref] [PubMed]
- Wong O. An industry wide mortality study of chemical workers occupationally exposed to benzene. I. General results. Br J Ind Med 1987;44:365-81. [Crossref] [PubMed]
- Fritschi L, Benke G, Hughes AM, et al. Risk of non-Hodgkin lymphoma associated with occupational exposure to solvents, metals, organic dusts and PCBs (Australia). Cancer Causes Control 2005;16:599-607. [Crossref] [PubMed]
- Xing C, Marchetti F, Li G, et al. Benzene exposure near the U.S. permissible limit is associated with sperm aneuploidy. Environ Health Perspect 2010;118:833-9. [Crossref] [PubMed]
- Grulich AE, Vajdic CM. The epidemiology of non-Hodgkin lymphoma. Pathology 2005;37:409-19. [Crossref] [PubMed]
- Schünemann H, Hill S, Guyatt G, et al. The GRADE approach and Bradford Hill's criteria for causation. J Epidemiol Community Health 2011;65:392-5. [Crossref] [PubMed]
- Maltoni C, Ciliberti A, Cotti G, et al. Benzene, an experimental multipotential carcinogen: results of the long-term bioassays performed at the Bologna Institute of Oncology. Environ Health Perspect 1989;82:109-24. [Crossref] [PubMed]
- Uzma N, Kumar BS, Hazari MA. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med 2010;53:1264-70. [Crossref] [PubMed]
- Hsieh GC, Sharma RP, Parker RD. Subclinical effects of groundwater contaminants. IV. Effects of repeated oral exposure to combinations of benzene and toluene on regional brain monoamine metabolism in mice. Arch Toxicol 1990;64:669-76. [Crossref] [PubMed]
- Ramírez-Lopera V, Uribe-Castro D, Bautista-Amorocho H, et al. The effects of genetic polymorphisms on benzene-exposed workers: A systematic review. Health Sci Rep 2021;4:e327. [Crossref] [PubMed]
- Spatari G, Allegra A, Carrieri M, et al. Epigenetic Effects of Benzene in Hematologic Neoplasms: The Altered Gene Expression. Cancers (Basel) 2021;13:2392. [Crossref] [PubMed]
- Sun Q, Wang B, Xu S, et al. Research development and trends of benzene-induced leukemia from 1990 to 2019-A bibliometric analysis. Environ Sci Pollut Res Int 2022;29:9626-39. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)