
Effect of taurine on the proliferation, apoptosis and MST1/Hippo signaling in prostate cancer cells
Introduction
Prostate cancer is among the most common malignancies of the male urinary tract. The latest epidemiological survey data from the International Agency for Research on Cancer in 2018 showed that prostate cancer accounted for 13.5% of all new cases of malignancies in men worldwide, with some patients dying from complications related to prostate cancer, which made it the 5th leading cause of cancer death in men worldwide (1). With increasing clinical research on prostate cancer, it has now been found that its development is associated with a variety of signaling abnormalities, among which mammalian sterilization of a 20-like kinase-1 (MST1) is aberrantly expressed in prostate cancer, and the Hippo signaling pathway to which MST1 belongs can induce the development of this disease (2). Although the mechanism of prostate cancer was identified, clinical treatment is still based on early surgery, radiotherapy and chemotherapy, which can control progression of the disease in the short term, but the 5-year survival rate of patients is still low. Taurine is a protective substance with multiple effects, and is thought to inhibit inflammatory responses, resist oxidative stress-induced damage and regulate immune disorders, as well as promoting apoptosis of cancer cells. Studies (3,4) have shown that taurine can inhibit tumor cell proliferation, promote apoptosis and induce autophagy. The PI3K/Akt/mTOR pathway is a central regulatory mechanism that regulates normal cell physiology and also plays a role in tumor genesis and metastasis. The inhibition of the PI3K/Akt/mTOR pathway can activate the autophagy of prostate cancer cells. MST1 plays a role in regulating cell cycle, promoting apoptosis and inhibiting tumor growth. Taurine is a sulfur-containing amino acid with simple chemical structure and is also a conditionally essential nutrient for human body. Taurine has a wide range of physiological effects, is the body's endogenous anti-injury substance. In recent years, it has been found that taurine has important application value in the prediction, prevention and treatment of tumors, and the concentration and transport of taurine in vivo are closely related to the occurrence and development of tumors. This study explored the mechanism of taurine in prostate cancer and provided important guidance for the clinical treatment of prostate cancer patients. Taurine, known chemically as 2-aminotaurine, is a sulfur-containing amino acid found in the body that has a simple chemical structure. The results of xenograft experiment in nude mice showed that the expression of anti-apoptotic protein Bcl-2 was significantly decreased and the expression of pro-apoptotic protein Bax was significantly increased after taurine treatment.
In this context, we selected a prostate cancer cell line and used a taurine intervention to analyze its effects on prostate cancer cell proliferation, apoptosis and the MST1/Hippo signaling pathway to provide a reference for subsequent clinical studies. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1297/rc).
Methods
Materials
The prostate cancer DU145 cell line was provided by Utilico (Shanghai) Life Sciences Ltd. (item No. YLK-XB127). The suppliers of the main were reagents were: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Nanjing KGI, China); taurine (Merck Sigma, USA); MST1 antibody, YAP antibody, Bax antibody, Bcl-2 antibody, horseradish peroxidase (HRP)-labeled secondary antibody (Abcam, USA).
Cell culture
The prostate cancer DU145 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) at 37 ℃ and trypsinized when the cells were 90% fused, after which they were passaged and logarithmically grown cells were harvested for subsequent analysis.
Analysis of taurine intervention concentrations
The rate of inhibition by taurine on the proliferation of prostate cancer DU145 cells at concentrations of 1, 10−1, 10−2, 10−3, 10−4, 10−5 and 10−6 mg/mL was determined using the MTT method. The cell concentration was adjusted to 3×104 cells/mL, and the suspension was prepared after the adjustment. Cells were inoculated in 96-well plates and incubated at 37 ℃ for 72 h, after which MTT reagent was added to obtain the cell optical density (OD) values and analyze the rate of inhibition by taurine: inhibition rate = (1 − OD value of taurine intervention wells)/OD value of blank wells × 100%. A quantitative-effect relationship curve was obtained and the taurine intervention concentration was determined after the calculation of the median lethal concentration (LC50) value for taurine was completed.
Grouping and intervention
The prostate cancer DU145 cells were divided into three groups: the blank group was cultured using conventional culture medium, the positive control group was cultured with 2 mg/mL cisplatin, and the taurine group was cultured using taurine intervention concentrations of 0.003 mg/mL as low, 0.03 mg/mL as medium and 0.3 mg/mL as high. The effect on prostate cancer DU145 cells at each concentration was observed after 72 h of culture.
Cell proliferation assay
Cells from each group were harvested and the cell proliferation rate was determined using the MTT method: cell proliferation rate = (OD of the cell group tested/OD of the control group) × 100%.
Measurement of apoptosis
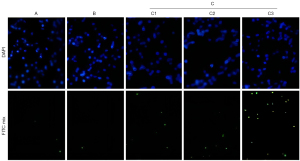
Cells from each group were harvested and the apoptotic rate was analyzed using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method: cells were washed, stained using 4',6-diamidino-2-phenylindole (DAPI), blocked and the nuclei color was observed using fluorescence microscopy, where green represents positive and blue represents negative. Apoptosis rate = number of positives/total cells × 100%.
MST1/Hippo signaling pathway protein assay
Cells from each group were harvested and analyzed for MST1/Hippo signaling pathway protein expression using western blot: 50 µg of the prepared protein was extracted and added to 2× SDS buffer, and after electrophoresis, membrane transfer, mold taking, fixation and closure, the MST1, YAP, Bax and Bcl-2 primary antibodies (1:1,000 TBST dilution) were incubated at 4 ℃, washed with TBST after 24h, HRP-labelled secondary antibody was added, the membrane was washed, and DAB was used as an internal reference to obtain MST1, YAP, Bax, Bcl-2 protein expressions.
Statistical analysis
The SPSS 26.0 statistical package was used. Measurement data are described as mean ± standard deviation (), analysis of variance was used for comparison among multiple groups, an independent sample t-test was used for comparison between groups, and P<0.05 was considered as a statistically significant difference.
Results
Taurine LC50 values, and experimental group determination
As shown in Table 1 and Figure 1, MTT analysis found that the rate of inhibition by taurine on proliferation of prostate cancer DU145 cells gradually decreased with each concentration, and its LC50 value was calculated as 0.3156 mg/mL. The dose-effect relationship curve was plotted, which showed that, combined with the LC50 value, the inhibition of prostate cancer DU145 cells was more significant at the low, medium and high concentrations of taurine set in this study as 0.003 mg/mL, 0.03 mg/mL and 0.3 mg/mL, respectively.
Table 1
| Taurine concentration (mg/mL) | OD value, | Inhibition rate (%) |
|---|---|---|
| 1 | 0.17±0.04 | 76.54 |
| 10−1 | 0.19±0.03 | 67.43 |
| 10−2 | 0.23±0.04 | 48.82 |
| 10−3 | 0.29±0.05 | 34.96 |
| 10−4 | 0.47±0.07 | 10.58 |
| 10−5 | 0.45±0.05 | 5.28 |
| 10−6 | 0.43±0.04 | 5.11 |
OD, optical density; , mean ± standard deviation.
Comparison of cell proliferation capacity between groups
As shown in Table 2 and Figure 2, the cell proliferation rates at 24, 48 and 72 h were lower in the positive control and taurine groups than in the blank group (P<0.05); the taurine group was lower than the positive control group (P<0.05); and the high concentration in the taurine group was lower than the medium concentration than the low concentration (P<0.05).
Table 2
| Group | Cell proliferation capacity (%) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Blank group, | 57.65±5.90 | 58.93±6.32 | 59.03±6.57 |
| Positive control group, | 43.21±4.76* | 38.92±4.39* | 25.31±2.39* |
| Taurine group, | |||
| Low concentration | 30.11±3.98*# | 27.60±2.10*# | 19.19±2.21*# |
| Medium concentration | 25.31±2.26*#▲ | 18.21±1.34*#▲ | 11.37±1.28*#▲ |
| High concentration | 18.53±1.15*#▲∆ | 10.48±0.69*#▲∆ | 5.32±0.65*#▲∆ |
*P<0.05; compared with the blank group, compared with the positive control group, #P<0.05; compared with the low-concentration taurine group, ▲P<0.05; compared with the medium-concentration taurine group, ∆P<0.05. , mean ± standard deviation.
Comparison of apoptotic capacity among groups
As shown in Table 3 and Figure 3, the apoptotic capacity of the cells was higher in the positive control group and taurine group than in the blank group (P<0.05); the taurine group was higher than the positive control group (P<0.05); and with the high concentration in the taurine group, it was higher than with the medium or low concentration (P<0.05).
Table 3
| Group | Apoptosis rate (%) |
|---|---|
| Blank group, | 7.43±1.12 |
| Positive control group, | 16.94±2.97* |
| Taurine group, | |
| Low concentration | 32.54±4.34*# |
| Medium concentration | 45.97±5.31*#▲ |
| High concentration | 55.58±6.43*#▲∆ |
*P<0.05; compared with the blank group, compared with the positive control group, #P<0.05; compared with the low-concentration taurine group, ▲P<0.05; compared with the medium-concentration taurine group, ∆P<0.05. , mean ± standard deviation.
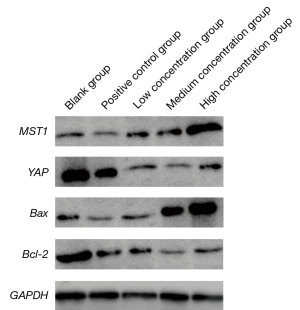
Comparison of MST1/Hippo signaling pathway proteins among groups
As shown in Table 4 and Figure 4, in terms of MST1 and Bax protein expressions, the positive control group and taurine group were higher than the blank group (P<0.05); the taurine group was higher than the positive control group (P<0.05); with the high concentration in the taurine group expression were higher than with the medium or low concentration (P<0.05); in terms of YAP and Bcl-2 protein expressions, the positive control group and taurine group were lower than the blank group (P<0.05); the taurine group was lower than the positive control group (P<0.05); and with the medium concentration in the taurine group expression were lower than with the low concentration (P<0.05).
Table 4
| Group | MST1 | YAP | Bax | Bcl-2 |
|---|---|---|---|---|
| Blank group, | 0.21±0.04 | 1.32±0.16 | 0.20±0.03 | 1.26±0.13 |
| Positive control group, | 0.56±0.06* | 0.99±0.11* | 0.43±0.05* | 1.00±0.10* |
| Taurine group, | ||||
| Low concentration | 0.79±0.08*# | 0.68±0.09*# | 0.76±0.08*# | 0.75±0.07*# |
| Medium concentration | 1.34±0.11*#▲ | 0.45±0.03*#▲ | 1.12±0.14*#▲ | 0.39±0.05*#▲ |
| High concentration | 1.68±0.23*#▲∆ | 0.20±0.05*#▲∆ | 1.64±0.23*#▲∆ | 0.18±0.03*#▲∆ |
*P<0.05; compared with blank group, compared with positive control group, #P<0.05; compared with low-concentration taurine group, ▲P<0.05; compared with medium-concentration taurine group, ∆P<0.05. MST1, mammalian sterilization of a 20-like kinase-1; , mean ± standard deviation.
Discussion
The age group affected by prostate cancer is becoming more and more youthful, and despite the current rapid advancements in medical technology, diagnostic tools and treatments that have achieved better results, the phenomenon of a low average survival period of patients still exists (5). Thus, it is particularly important to actively search for drugs that can effectively treat prostate cancer and prolong the life span.
Taurine is the main active ingredient in calculus bovis, which is mainly distributed in the reproductive organs, kidney, heart and brain of the human body, and is involved in a variety of physiological activities (6,7). It has been reported that taurine can regulate oxidative stress, suppress immune disorders and repair liver damage (8,9). Many clinical studies have been devoted to the role of taurine, such as Li et al., who suggested that taurine induces apoptosis of cervical cancer cells (10), and He et al., who also found that taurine induced apoptosis in cancer cells (11). Our results showed that the proliferation rate of cells cultured with taurine decreased and the apoptosis rate increased significantly, and the effect was strongest at high concentrations of taurine. These results suggested that taurine can dose-dependently inhibit the proliferation of prostate cancer cells and promote apoptosis, similar to the published results.
The Hippo signaling pathway is a recently discovered pathway that regulates cell growth. Currently, study is directed at the relationship between the Hippo signaling pathway and cell proliferation and apoptosis, and the pathway is believed to have a role in promoting lung cancer, prostate cancer, colorectal cancer and other tumors (12,13). The core kinase chain of the Hippo signaling pathway is MST1, with YAP/TAZ as the downstream target (14). When the Hippo signaling pathway is activated, MST1 induces YAP phosphorylation in the presence of associated scaffolding proteins. When phosphorylated YAP accumulates in the cytoplasm, it is rapidly degraded by ubiquitination, thus exerting its effect of inducing apoptosis and inhibiting cell proliferation (15-17). Wang et al. found that FFAR1 and FFAR4-dependent Hippo pathway activation mediated DHA-induced apoptosis in androgen non-dependent prostate cancer cells (18). Salem et al. similarly found that the Hippo signaling pathway was involved in aberrant proliferation of prostate cancer cells (19). A previous study has found that MST1 is highly conserved and its aberrant expression is associated with a variety of malignancies such as colorectal and hepatocellular carcinomas through its role in inducing cancer cell proliferation and apoptosis (20). YAP, on the other hand, is a candidate oncogene and is highly expressed in many types of human tumors (21). In the present study, we found that the expression of MST1, a key protein in the MST1/Hippo signaling pathway, was increased and the expression of YAP protein was decreased in the groups of cells with the taurine intervention at different concentrations.
Taurine can promote the secretion of pituitary hormone, activate the function of pancreas, so as to improve the state of the body’s endocrine system, beneficial regulation of body metabolism, and has the function of promoting the organism immunity enhancement and anti-fatigue. Taurine can promote the recovery of acute hepatitis; Taurine had protective effect on the changes of rabbit primary renal tubular epithelial cells induced by cisplatin. Taurine has the function of inhibiting platelet agglutination, enhancing myocardial contractility and lowering blood pressure in blood circulation. Chemotherapy will have certain damage to the viscera, taurine can alleviate the damage caused by chemotherapy drugs. In addition, studies have found that taurine can inhibit cell proliferation and promote cell apoptosis in colorectal cancer, and taurine can inhibit cardiomyocyte apoptosis induced by adriamycin by up-regulating the activity of PI3K/Akt signaling pathway and down-regulating the activities of P53 and P38-JNK (22,23). Taurine can also induce autophagy and apoptosis of PC12 cells by activating mTOR signaling pathway and inhibiting methamphetamine.
In summary, taurine promoted apoptosis and inhibited proliferation of prostate cancer cells, and its mechanism of action may be related to the MST1/Hippo signaling pathway, acting in a dose-dependent manner. Although this study showed that taurine can inhibit the proliferation of prostate cancer cells, it needs to be further verified in patients with prostate cancer in order to improve the prognosis of prostate cancer patients.
Acknowledgments
Funding: This work was supported by the 2019 Guangdong Medical Research Fund Project (No. A2019309); 2018 Guangzhou Health and Family Planning Technology General Guidance Project (No. 20181A011071); Key Discipline Construction Project of Pudong Health and Family Planning Commission of Shanghai (No. PWZxk2017-19).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1297/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1297/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1297/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Hao L, Li H, Zhang S, et al. Integrative Exome Sequencing Analysis in Castration-Resistant Prostate Cancer in Chinese Population. Curr Pharm Biotechnol 2020;21:140-8. [Crossref] [PubMed]
- Zhu Y, Freedland SJ, Ye D. Prostate Cancer and Prostatic Diseases Best of Asia, 2019: challenges and opportunities. Prostate Cancer Prostatic Dis 2020;23:197-8. [Crossref] [PubMed]
- Wu P, Chen X. Taurine Ameliorates High Glucose Induced Apoptosis in HT-22 Cells. Adv Exp Med Biol 2019;1155:889-903. [Crossref] [PubMed]
- Natale C, Carlos C, Hong J, et al. Testosterone Therapy After Prostate Cancer Treatment: A Review of Literature. Sex Med Rev 2021;9:393-405. [Crossref] [PubMed]
- Dayang W, Dongbo P. Taurine prevents ultraviolet B induced apoptosis in retinal ganglion cells. Cutan Ocul Toxicol 2018;37:90-5. [Crossref] [PubMed]
- Wu P, Shi X, Luo M, et al. Taurine inhibits neuron apoptosis in hippocampus of diabetic rats and high glucose exposed HT-22 cells via the NGF-Akt/Bad pathway. Amino Acids 2020;52:87-102. [Crossref] [PubMed]
- Inam-U-Llah. Protective Effect of Taurine on Apoptosis of Spinal Cord Cells in Diabetic Neuropathy Rats. Adv Exp Med Biol 2019;1155:875-87. [Crossref] [PubMed]
- Tu S, Zhang XL, Wan HF, et al. Effect of taurine on cell proliferation and apoptosis human lung cancer A549 cells. Oncol Lett 2018;15:5473-80. [Crossref] [PubMed]
- Li H, Ruan WJ, Liu LQ, et al. Impact of Taurine on the proliferation and apoptosis of human cervical carcinoma cells and its mechanism. Chin Med J (Engl) 2019;132:948-56. [Crossref] [PubMed]
- He F, Ma N, Midorikawa K, et al. Taurine exhibits an apoptosis-inducing effect on human nasopharyngeal carcinoma cells through PTEN/Akt pathways in vitro. Amino Acids 2018;50:1749-58. [Crossref] [PubMed]
- Haiping C, Qi X, Dawei L, et al. Citron Rho-interacting serine/threonine kinase knockdown suppresses prostate cancer cell proliferation and metastasis by blocking Hippo-YAP pathway. Nan Fang Yi Ke Da Xue Xue Bao 2019;39:257-63. [PubMed]
- Li Q, Wang M, Hu Y, et al. MYBL2 disrupts the Hippo-YAP pathway and confers castration resistance and metastatic potential in prostate cancer. Theranostics 2021;11:5794-812. [Crossref] [PubMed]
- Taha Z, Janse van Rensburg HJ, Yang X. The Hippo Pathway: Immunity and Cancer. Cancers (Basel) 2018;10:94. [Crossref] [PubMed]
- Bainbridge A, Walker S, Smith J, et al. IKBKE activity enhances AR levels in advanced prostate cancer via modulation of the Hippo pathway. Nucleic Acids Res 2020;48:5366-82. [Crossref] [PubMed]
- Wang XY, Xiao J, Ma HQ. Two new Cu(II)-based coordination polymers: inhibitory activity on prostate cancer by reducing EGF-R expression and HIPPO signaling pathway activation. J Biomater Sci Polym Ed 2020;31:1741-55. [Crossref] [PubMed]
- Matsuda Y, Narita S, Nara T, et al. Impact of nuclear YAP1 expression in residual cancer after neoadjuvant chemohormonal therapy with docetaxel for high-risk localized prostate cancer. BMC Cancer 2020;20:302. [Crossref] [PubMed]
- Wang J, Hong Y, Shao S, et al. FFAR1-and FFAR4-dependent activation of Hippo pathway mediates DHA-induced apoptosis of androgen-independent prostate cancer cells. Biochem Biophys Res Commun 2018;506:590-6. [Crossref] [PubMed]
- Salem O, Hansen CG. The Hippo Pathway in Prostate Cancer. Cells 2019;8:370. [Crossref] [PubMed]
- Cui J, Zhou Z, Yang H, et al. MST1 Suppresses Pancreatic Cancer Progression via ROS-Induced Pyroptosis. Mol Cancer Res 2019;17:1316-25. [Crossref] [PubMed]
- Chen C, Yuan W, Zhou Q, et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics 2021;11:4298-315. [Crossref] [PubMed]
- Chen CX, Wan HF, Li SY, et al. The inhibition of the invasion and metastasis of colorectal cancer cells by taurine through regulation of the PTEN/AKT/GSK-3beta pathway. Asian J Surg 2022;45:970-3. [Crossref] [PubMed]
- Liu Z, Xia Y, Zhang X, et al. Roles of the MST1-JNK signaling pathway in apoptosis of colorectal cancer cells induced by Taurine. Libyan J Med 2018;13:1500346. [Crossref] [PubMed]
(English Language Editor: K. Brown)




