
Single incision-plus approach for gasless endoscopic parotidectomy: a seven-step procedure
Introduction
Parotid neoplasms, one of the most common salivary gland diseases, are typically treated with surgical excision. Conventional surgery requires lifting of a large fascial tissue flap for adequate surgical field exposure via an S-shaped or Y-shaped incision (1). For aesthetic, the incision length is gradually shortened from facelift to periauricular incision, and endoscopic parotidectomy emerged (2). In 2000, endoscopic parotidectomy was first described by Lin et al. (3), and recently this developed endoscope-assisted parotidectomy progress from a forepassed incision to various ultra-modern designs. Then in 2009, Sun et al. recognized and preliminarily explored it as two small skin incisions (4). The two-incision approach was superseded rapidly due to the obvious additional scar below the jaw, so it was converted into a single retroauricular incision and adopted to become mainstream until now. Compared with conventional resection, this procedure results in a better aesthetic and functional postoperative outcome (5), as well as less intraoperative bleeding, lower incidences of temporary facial paralysis and Frey’s syndrome, and higher postoperative satisfaction (6). At one point, it appeared that the incision within the postauricular groove was the only option for endoscopic parotidectomy. Fortunately, Woo et al. contributed a new approach to endoscope-assisted parotid surgery via hairline incision, which is also used in endoscopic submandibular gland excision (7). In comparison to the retroauricular incision, the hairline incision leaves no scar on the face, making it the best choice. Current endoscope-assisted parotidectomy is only appropriate for benign and low-grade malignant tumors located in the tail of the parotid gland; otherwise, a longer incision is required. On this basis, we developed a new “plus incision” to expand indication.
Endoscopic parotidectomy is still in its infancy when compared to the mature application of endoscopic operational techniques in thyroidectomy. Mostly, endoscope only serves as magnifying glass to aid in simply visualizing the key structure (8). Besides, incision design is required to be easily converted to the conventional approach due to its difficulty (9). Endoscopic parotidectomy is enduring the absence of imperative standard in incision design, anatomic summary, and surgical steps. In the current study, we summarize previous literature experiences and contribute our clinical exploration of anatomical precautions, feasible instruments, and surgical procedures under single incision-plus in gasless endoscopic parotidectomy, called seven-step method.
Surgical techniques
Study design and participants
The current study has been approved by the Ethics Committee of West China Hospital of Stomatology, Sichuan University (Approval No. WCHSIRB-D-2020-311-R1). All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). The tumor’s malignancy was assessed using contrast-enhanced CT and color Doppler ultrasound. Patients with benign and low-grade malignant tumors were eligible for endoscopic parotidectomy, whereas patients with high-grade malignant and recurrent tumors were not. Each patient made choices voluntarily and signed the informed consent forms after knowing about the procedures’ benefits and drawbacks. The consent form is used to inform patients about surgical procedures and the use of their data.
STEP 1: preoperative preparation
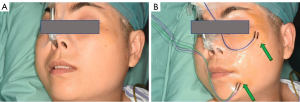
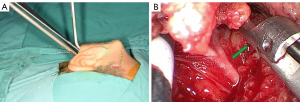
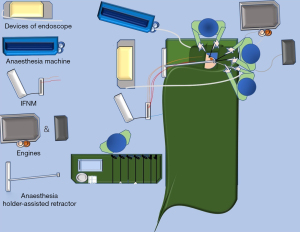
Each patient’s preoperative hair removal involves 2 cm of the temporal region of the scalp above the hairline and half of the occipital region of the affected side behind the hairline. Under general endotracheal anesthesia and no muscle relaxant, the patient is placed in a supine position with the shoulders raised by a surgical drape and the head tilted nearly 90o to the healthy side (Figure 1A). For more efficient observation, the display devices of endoscope and intraoperative facial nerve monitoring (IFNM) are placed on the healthy side of patient near the operating table. In the same direction, an anesthesia holder-assisted retractor is placed, whereas the engines of the ultrasonic scalpel, aspirator and electrocautery are placed on the affected side. Following topical skin disinfection, the positive terminals of IFNM are inserted into the subcutaneous tissue of the manubriosternal region, whereas the searching units of IFNM are pierced into the orbicularis oculi and orbicularis oris respectively (Figure 1B). Usually, the monitor’s event threshold for triggering the alarm was set at 100 mV, with stimulation parameters ranging between 0.8 and 2.0 mA of constant current stimulation. Correct steps should be followed to ensure that the opposite ends of above-mentioned units are properly connected, and some viscous eye masks should be placed over these puncture points to avoid unnecessary shifts.
The surgical region then is disinfected routinely and draped with sterilized towels. Nasal endoscopy is chosen for optical image acquisition because of its 4-millimeters caliber. The white balance calibration should be checked, and the focus should be well adjusted, so that vessels and nerves are recognizable on the viewing screen. Ultrasonic scalpel, aspirator, electrocautery, bipolar coagulator, and stimulating probe of IFNM are properly assembled. All surgical endoscopic instruments are technical instruments with long structures designed to fit the cavity-type surgical field that are rarely seen in the major reports of endoscopic parotidectomy.
The surgical team consists of a chief surgeon, an endoscope assistant, a scrub nurse, and an additional assistant surgeon. It is worth mentioning that the additional assistant surgeon assists by using endoscopic instruments through the plus-incision. Through this plus-incision, objects like blood can be sucked away to create a clean operating space, or the delaminated superficial tissue can be lifted to maintain stable exposure of deep structures. This division of surgeons also contributes to relieve the “chopstick” effect caused by the parallel arrangement of the instruments in the cavity (10), since the orthogonal passage is thought to be an easy way to carry out delicate operations.
STEP 2: design of retroauricular-hairline incision and plus-incision
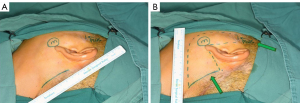
Surgical incisions should be designed in two regions. The main one is made at retroauricular hairline, while the “plus” one is made within the hairline in front of the tragus. Optionally, a line is drawn from the ear lobe to the retroauricular hairline over mastoid process, with the intersection point forming the superior end of main incision, which extends downward along the hairline. The inferior end of the hairline incision is determined by drawing a horizontal line from the mandibular angle to the retroauricular hairline (Figure 2A). Then, the body surface reflection of the neoplasms is marked precisely. Designing the surgical field on the skin in advance will guide us to elevate the flap in the correct direction. Eventually, a regular quadrilateral field of endoscopic surgery will appear to be marked (Figure 2B).
STEP 3: surgical cavities creation and coalescence
Plus-incision may proceed cavity separation depending on the preoperative design. Incise through the skin and subcutaneous layers until the superficial temporal fascia is exposed, avoiding deep dissection. Blunt dissection under the subcutaneous layer above the superficial fascia is a safe and simple procedure (11), because the dense collagen fibers of the superficial temporal fascia are clear as inferior boundaries, which helps avoid injury to facial nerve zygomatic branch. When skilled, a rectangular-shaped cavity is easily and quickly created at superficial layer of parotid masseter fascia, starting with “plus” incision and ending with the neoplasm (Figure 3A). The advantage of plus cavity is the distinct demarcation of the surgical prezone at subcutaneous fatty layer. In addition, plus cavity aids in the separation of the superficial musculoaponeurotic system (SMAS) from the dermis to avoid anatomical hierarchy error, reducing the possibility of skin piercing during the creation of the main cavity.
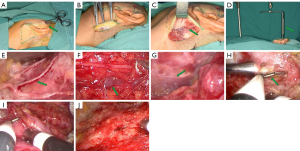
The main surgical cavity is created in two stage process, starting with the conventional method of lifting the flap from retroauricular hairline and then expanding the cavity by an endoscope-assisted approach until it merges with the plus-incision cavity. Under direct vision, incise through the skin, subcutaneous fatty layer, and SMAS. SMAS can be easily lifted by general surgical instruments, and separated from deep fatty layer to form the prime cavity (Figure 3B). Due to the close connection between the sternocleidomastoid muscle and the SMAS in this area, sharp dissection is carried out using electrocautery while preserving the sternocleidomastoid muscle’s integrity. The great auricular nerve, a branch of the cervical plexus nerve, usually crosses the sternocleidomastoid muscle and should be carefully protected to reduce postoperative numbness in the auricular region (Figure 3C). To avoid causing any damage to the encountered great auricular nerve, it is best to avoid electrocautery dissection. Following this stage, we will enter the real endoscopic stage, in which we transition from traditional surgery to true endoscopic approach. Operational tunnel will be lifted by the anesthesia holder-assisted retractor and accommodate three instruments, including a nasal endoscope, an ultrasonic scalpel, and an endoscopic aspirator (Figure 3D). The endoscope assistant keeps the 0° nasal endoscope photographing while the chief surgeon holds the ultrasonic scalpel and endoscopic aspirator. Without gas filling, the anatomical structures are shown as clear images on the screen. The anesthesia holder-assisted retractor is adjusted to continue the dissection of the sternocleidomastoid muscle. Following dissection of the great auricular nerve (Figure 3E), posterior auricular vein is observed after bypassing the great auricular nerve and is dissociated from the fatty layer in order to release it (Figure 3F). While the anteroinferior surface of cavity may change from SMAS to platysma, the anterior border of sternocleidomastoid muscle may be hidden by adipose tissue, whereas the red muscle bundles of platysma are easily distinguished (Figure 3G). At this stage we will follow platysma instead of following sternocleidomastoid muscle. The cavity is then extended, and the SMAS in the anterior and superior boundaries of the cavity is dissected until meet the "plus" cavity (Figure 3H), resulting in coalescing of the two cavities. The cavity is then extended and the superficial parotid gland is sufficiently exposed. (Figure 3I,3J).
STEP 4: separation of surgical boundaries
Following the creation of the surgical cavity, more defined surgical boundaries are required, which can be divided into six three-dimensional boundaries: (I) the anterior boundary that is bounded by the transition from parotid gland to masseter muscle; (II) the posterior boundary that is formed by the anterior border of sternocleidomastoid muscle; (III) the superior boundary that is bounded by the cartilage of external acoustic meatus; (IV) the inferior boundary that is formed by the inferior margin of mandible; (V) the top boundary that is formed by the platysma and skin; (VI) the bottom boundary, which is bounded by the facial nerve for superficial parotid lobectomy and the styloid process of temporal bone for deep parotid lobectomy.
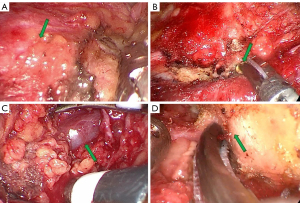
Separate platysma muscle from parotid masseter muscle fascia to access the transition from parotid gland to masseter muscle (Figure 4A). Then, hold the endoscopy back to focus on sternocleidomastoid muscle. The anterior border of sternocleidomastoid muscle may be hidden by adipose tissue, which is then lifted and opened (Figure 4B). After that, the anterior border of sternocleidomastoid muscle is separated and completely exposed until the retromandibular vein is visible (Figure 4C). The retromandibular vein should be protected because it will not be involved in the surgery. Usually, the superficial parotid gland dissection doesn’t encounter large vein branches, so the retromandibular vein serves as a warning sign that this region is deep and may be close to the styloid process. It is not recommended to perform further superior tissue dissection in an attempt to follow the retromandibular vein towards superior. At this stage, facial nerve trunk may be encountered. However, parotid gland can be safely opened at superficial region of the mastoid. Meanwhile, the connection between the parotid gland and the cartilage of the external acoustic meatus can be severed partially using ultrasonic scalpel for better tissue dissociation, allowing access to the facial nerve trunk (Figure 4D).
STEP 5: separation and protection of the facial nerve trunk
Protection of facial nerve has the priority in parotidectomy, especially for benign and low-grade malignant tumors. There is no evidence that there is a significant difference in postoperative outcomes between antegrade and retrograde facial nerve dissection in benign parotid surgery (12). However, the precise location of the facial nerve in relation to palpable or visible facial landmarks is variable (13). The two-dimensional branching patterns of the marginal and cervical branches of the facial nerve are quite variable (14). this is the reason why we chose the antegrade facial nerve dissection and why the current procedure does not sufficiently separate the inferior boundary. At this stage, change the position of surgeons so that an ultrasonic scalpel, aspirator and endoscope can be used to follow a 45° line from the hairline incision across the mastoid to deep (Figure 5A). Facial nerve trunk always exists at the extension of this 45° line (Figure 5B). To reduce the risk of bleeding, use an ultrasonic scalpel intermittently at multiple points to hold a small amount of tissue, and use ultrasonic coagulation to stop bleeding from the encountered retromandibular vein capillaries or a bipolar coagulator can be used in some situations to perform better cauterization. In the case of the extensive errhysis, adrenalin wet sliver can be packed for a brief period of time. Under endoscope magnification in a clean surgical field, the facial nerve trunk will be as distinct as jade.
STEP 6: processing of the branches of facial nerve
After the facial nerve trunk has been clearly separated, the superficial parotid gland above the facial nerve can be cut more safely with an ultrasonic scalpel. Perform an anterograde blunt dissection to expose each branch of the facial nerve using IFNM to aid in nerve identification by placing the IFNM stimulating probe on the nerve-like structure. When the zygomatic and temporal branches of the facial nerve are electrically stimulated, the signal of the orbicularis oculi muscle is collected (Figure 6A), whereas the signal of the orbicularis oris muscle is collected when the marginal mandibular branch of the facial nerve is electrically stimulated (Figure 6B), and both signals are collected when the trunk is stimulated (Figure 6C). The zygomatic and temporal branches of the facial nerve run close to the cartilage of the external auditory canal and make a circuitous upward turn (Figure 6D). It is not necessary to dissect these two branches of the facial nerve when tumor is located at the tail of the parotid gland. However, for tumors located in front of the tragus, care should be taken during the blunt dissection of the zygomatic and temporal branches. Through the “Plus” cavity, parotid tissues near the tragus can be pulled or pushed, allowing tumors located more superiorly, even in accessory parotid gland, to be turned and resected as far as possible at the main cavity. Protect the marginal and cervical branches of the facial nerve and dissect the inferior boundary until it is sufficiently separated (Figure 6E). After that lift the entire superficial lobe of the parotid gland from back to front, paying special attention to the marginal mandibular branch of the facial nerve, which gradually rises from the depths to become more superficial. Adrenaline wet sliver can be used to push tissue, improving the vision, and reducing bleeding. Tissues are more difficult to lift in the surgical prezone, resulting in a severe chopstick effect. To avoid the chopstick effect, use the instrument skillfully through the "Plus" incision to create a situation in which instruments are interlaced.
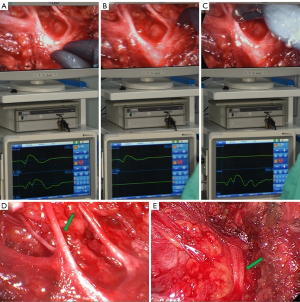
Conventional opinions agree that the unwise manipulation of the parotid duct may result in salivary leakage. The functional superficial parotidectomy is the most widely used procedure for removing benign and low-grade malignant tumors from the superficial lobe of parotid gland. Because a functional superficial parotidectomy can preserve salivary function by preserving the Stensen’s duct, it makes no difference whether the parotid duct is ligated or not in this case. However, because the major branches of the parotid duct from the resected site are not ligated, salivary leakage remains a possibility (15). If the parotid duct is not ligated, postoperative parotid area bandage compression can help prevent salivary leakage. To avoid unnecessary nerve injury, the parotid duct should be distinguished from the facial nerve, particularly the upper or lower buccal branch of the facial nerve.
STEP 7: en bloc resection of the superficial parotid gland and tumor
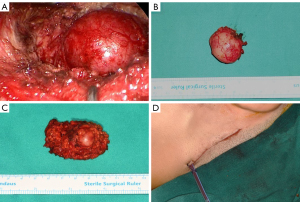
With repeated observation of the facial nerve position, the superficial lobe of the parotid gland and its tumor are gradually separated (Figure 7A). Cut the remaining fascia connection at the junction of the parotid gland and masseter muscle until the parotid gland and tumor are completely excised and removed as a biopsy specimen through the retroauricular hairline incision (Figure 7B,7C). To properly clean the surgical site, the operational cavity is irrigated with normal saline. It is recommended to irrigate through the "Plus" incision to the hairline incision for additional cleaning. After irrigation of the surgical site, all branches of the facial nerve should be easily identified, and IFNM can be used to assess neural integrity. To stop the bleeding, the bleeding spots are detected and properly hemostasised using bipolar electrocoagulation. After inserting a drainage tube through the inferior order of hairline incision, incisions are sutured layer by layer, and a bandage is applied to the parotid area (Figure 7D).
Comments
In the current descriptive study, we summarize the gasless endoscopic parotidectomy through single incision-plus by seven-step method. Our experience has led us to the following precautions, which we have concluded: (I) Preoperative preparation should not be underestimated. (II) Technical endoscopic instruments must be properly assembled to avoid wasting time. (III) IFNM may help decrease the risk of immediate post-operative and permanent facial nerve weakness in primary parotid gland surgery (16). (IV) A 4.5cm single incision in the hairline can also result in a successful surgery. However, the 6 cm long incision provides a better surgical experience. “Plus” incision is useful in difficult situations, especially when the chopstick effect occurs. (V) The two-stage process of cavity creation is recommended to be demarcated by great auricular nerve. Choosing great auricular nerve as change indicator is for higher preservation possibilities which lead to better satisfaction and lower risk of medical disputes (17). (VI) Because clear boundaries are more important in surgical cavities, do not try to locate the facial nerve before determining the boundary. (VII) Because the 45°-line method for searching facial nerve trunk is simple and effective, antegrade facial nerve dissection is logical and recommended. (VIII) As the depth of the operation increases, tissue traction becomes more difficult and space becomes more limited. (IX) Postoperative high-quality bandage with pressure is necessary.
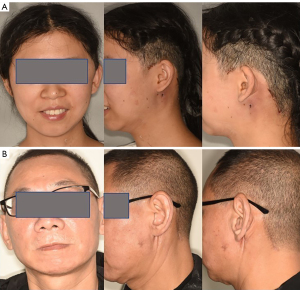
Endoscopic parotidectomy is technically demanding procedure. The learning curve of time and frequency is influenced by many factors, like anatomy, instruments, procedures and patience. Preoperative evaluation of the surgical region using contrast-enhanced CT and color Doppler ultrasound is recommended, especially for the deep lobe of parotid gland. The seven-step method is indicated for benign and low-grade malignant tumors in parotid gland and masseter muscle. With the accumulation of experience and operation proficiency of surgeons, tumors of both surficial lobe and deep lobe can be removed smoothly. However, the method is more difficult to be used in recurrent tumors. When the layers are difficult to be distinguished due to tissue adhesion, open method may be more convenient. In future, more developed oral and neck endoscopic instruments are required to be used in clinical practice. Finally, for more knowledge, see Figures 8,9, where Figure 8 shows the outcome after a 1-month follow-up and Figure 9 illustrates the organization of the operating room and the gasless retroauricular-hairline endoscopic parotidectomy assembly.
Conclusions
The seven-step method, which is based on a single incision-plus, is expected to be a mature operational procedure capable of not only visualizing key structures but also producing an aesthetically pleasing effect. This technique, we believe, deserves to be promoted as a method of teaching gasless endoscopic parotidectomy.
Acknowledgments
Funding:
Footnote
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-226/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-226/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The current study has been approved by the Ethics Committee of West China Hospital of Stomatology, Sichuan University (Approval No. WCHSIRB-D-2020-311-R1). All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). Each patient made choices voluntarily and signed the informed consent forms after knowing about the procedures’ benefits and drawbacks.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moori PL, Rahman S. Endoscopic versus conventional parotid gland excision: a systematic review and meta-analysis. Br J Oral Maxillofac Surg 2021;59:272-80. [Crossref] [PubMed]
- Roh JL. Gland-preserving surgery of benign parotid tumours via postauricular sulcus incision: Is it safe and effective with the scarless incision? Oral Oncol 2022;127:105808. [Crossref] [PubMed]
- Lin SD, Tsai CC, Lai CS, et al. Endoscope-assisted parotidectomy for benign parotid tumors. Ann Plast Surg 2000;45:269-73. [Crossref] [PubMed]
- Sun W, Xu YD, Zheng YQ, et al. Endoscope-assisted partial-superficial parotidectomy through two small skin incisions. Acta Otolaryngol 2009;129:1493-7. [Crossref] [PubMed]
- Gao L, Liang QL, Ren WH, et al. Comparison of endoscope-assisted versus conventional resection of parotid tumors. Br J Oral Maxillofac Surg 2019;57:1003-8. [Crossref] [PubMed]
- Zou HW, Gao J, Liu JX, et al. Feasibility and advantages of endoscope-assisted parotidectomy: a systematic review and meta-analysis. Br J Oral Maxillofac Surg 2021;59:503-10. [Crossref] [PubMed]
- Woo SH, Kim JP, Baek CH. Endoscope-assisted extracapsular dissection of benign parotid tumors using hairline incision. Head Neck 2016;38:375-9. [Crossref] [PubMed]
- Chen MK, Chang CC. Minimally invasive endoscope-assisted parotidectomy: a new approach. Laryngoscope 2007;117:1934-7. [Crossref] [PubMed]
- Li T, Liu Y, Wang Q, et al. Parotidectomy by an endoscopic-assisted postauricular-groove approach. Head Neck 2019;41:2851-9. [Crossref] [PubMed]
- Wang Y, Yao Y, Dou Y, et al. Chopstick technique used in laparoendoscopic single site radical hysterectomy for early stage cervical cancer. Sci Rep 2021;11:6882. [Crossref] [PubMed]
- Hînganu D, Stan CI, Ţăranu T, et al. The anatomical and functional characteristics of parotid fascia. Rom J Morphol Embryol 2017;58:1327-31. [PubMed]
- Mashrah MA, Al-Dhohrah TA, Al-Zubeiry FA, et al. Antegrade versus retrograde facial nerve dissection in benign parotid surgery: Is there a difference in postoperative outcomes? A meta-analysis. PLoS One 2018;13:e0206028. Erratum in: PLoS One 2020;15:e0242299. [Crossref] [PubMed]
- Roostaeian J, Rohrich RJ, Stuzin JM. Anatomical considerations to prevent facial nerve injury. Plast Reconstr Surg 2015;135:1318-27. [Crossref] [PubMed]
- Stuzin JM, Rohrich RJ. Facial Nerve Danger Zones. Plast Reconstr Surg 2020;145:99-102. [Crossref] [PubMed]
- Chang JW, Leem SS, Choi HJ, et al. Modified Functional Superficial Parotidectomy With Ligation of the Major Branch of the Parotid Duct Extending to the Superficial Lobe. Ann Plast Surg 2017;78:507-10. [Crossref] [PubMed]
- Chiesa-Estomba CM, Larruscain-Sarasola E, Lechien JR, et al. Facial nerve monitoring during parotid gland surgery: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2021;278:933-43. [Crossref] [PubMed]
- Xu C, Sun Q. Great auricular nerve schwannoma in neck region: a case report with the risk of medical disputes. BMC Neurol 2019;19:308. [Crossref] [PubMed]


