
Tumor-treating fields in combination with sorafenib curtails the growth of colorectal carcinoma by inactivating AKT/STAT3 signaling
Introduction
Colorectal carcinoma (CRC) is the fourth most frequent type of cancer across the world. In 2008, CRC’s estimated incident stood at 436,000 cases in Europe, causing over 212,000 deaths (1). In addition, in spite of the commendable improvements made in screening as well as early disease management, nearly 30% of patients have synchronous metastases whereas 50% to 60% of them tend to experience metastases that inevitably necessitates chemotherapy. Existing management is premised on several active drugs [5-fluorouracil (5-FU)/leucovorin (LV), oxaliplatin, irinotecan, cetuximab, panitumumab and bevacizumab] either as single agents or as combination (2). Due to such treatments, there has been an overall improvement rate in patients’ survival, although tumor resistance continues to be a common reason for chemotherapy failure.
In this regard, tumor-treating fields (TTFields) denote intermediate frequency, low intensity, and alternating electric fields supplied via noninvasive transducer arrays that are situated in a locoregional manner in close proximity to the tumor’s anatomic region. Even as that biological impacts of TTFields are still being made, the section explicates existing knowledge about the action mechanism. As immensely polar dipoles, tubulin dimers straighten with the direction of TTFields’ application. It has also been shown that TTFields are capable of disrupting the polymerization action of microtubules which generate the mitotic spindle, by eradicating the tubulin subunits’ typical randomized motion in the cytoplasm during metaphase (3,4). In turn, this causes metaphase arrest, prolonged mitosis, and eventually, death of cells. Phase 3 trial showed the efficacy of this modality alone equivalent compared to chemotherapy (5). Novel therapies such as immune modulatory therapies and electrical field treatment could improve survival (6-9). Future advances in molecular biology and nanotechnology may hopefully give better results for the management of this grad 4 disease (10).
It has mainly been found that they are able to increase survival rates in patients suffering from glioblastoma multiforme (GBM), which then resulted in its permission from the FDA for newly diagnosed and recurrent GBM after radiotherapy and surgery with adjuvant temozolomide (11,12). TTFields is currently considered as a category 2A treatment for patients with a good performance status, according to the most recent guidelines of National Comprehensive Cancer Network (NCCN) (13). Various kinds of cancers were used as platforms to verify the effectiveness of TTFields in preclinical studies, including colorectal, hepatocellular, melanoma, renal, breast, gastric, cervical, small lung cancer, and urinary transitional cell as well (14,15).
As an oral multikinase inhibitor, sorafenib (Nexavar) has gained the approval from the U.S. Food and Drug Administration (FDA) for treating patients with advanced renal cell carcinoma (RCC) as well as those with unresectable hepatocellular carcinoma (HCC). The European Medicines Agency (EMA) has also approved it for treating patients with HCC and with advanced RCC on whom previous IFN-α or interleukin-2-based therapy had failed or those that were deemed unfeasible for this therapy. Sorafenib is a multi-targeted tyrosine kinase inhibitor (TKI) whose antiangiogenic properties are primarily blocked by vascular endothelial growth factor-receptor (VEGFR) and the activation of platelet-derived growth factor receptor (PDGFR) (16-18). Furthermore, it is known to regulate the Raf-MEK-ERK pathway by curtailing C- and B-Raf. Recently, admitting the targeted therapy such as bevacizumab, cetuximab, panitumomab and Sorafenib to the cytotoxic therapy has provided survival advantage in patients with advanced stage CRC (19-21).
This study aimed to evaluate and assess the bio-activity of Sorafenib in therapy that employs TTFields as a TTFields-sensitizer on CRC. Our study intends to undergird the relevance and efficaciousness of multimodal therapy with TTFields + sorafenib combination therapy for CRC using empirical and scientific evidence, as was previously noted in the study of TTFields + 5-FU combined therapy (22). We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1853/rc).
Methods
Experimental setting of the device
TTFields were produced using a pair of insulated wires connected to a function generator and a high-voltage amplifier that produced sine-wave signals ranging from 0 to 800 V (23). To treat the TTFields to cell lines, a pair of insulated wires was installed to the lowest part of each cell dish, 1 cm from each other. The treated TTFields intensity and frequency were 0.9–1.5 V/cm and 150 kHz.
Antibodies and chemicals
Antibodies to p-p53, cleaved caspase-3, p-STAT3 and p-AKT were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies to p53, BCL-2, beta-actin, total STAT3 and total AKT were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Stattic hydrochloride (>95%) which is a potent STAT3 inhibitor and inhibits STAT3 phosphorylation (at Y705 and S727) was purchased from Sigma-Aldrich (19983-44-9). LY294002 was purchased from Cell Signaling Technology (#9901) (Boston, MA, USA).
Cell culture
Human HCT116 and SW480 CRC cell lines were acquired from the American Type Culture Collection (ATCC, Manassas, VA, USA). HCT116 and SW480 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (both Gibco; Thermo Fisher Scientific, Germany), 0.1 mM non-essential amino acids, glutamine, HEPES and penicillin (100−1,100 U/mL) at 37 ℃ in a 5% CO2 humidified incubator.
Trypan blue cell counting assay
Cell viability was determined by trypan blue exclusion assay. To a cell suspension, and equal volume of trypan blue reagent was added. This was followed by the microscopic evaluation of the rate of viable cells. All assays performed were in triplicate.
Cell-viability assay
Cells were set at a confluency of 5×103 cells per well in a 96-well plate and incubated for 24 h. To assess cell viability, same volume of culture media including EZ-Cytox (EZ3000, Daeillab Service) was added to each well, and the wells were incubated for 4 h. The cell viabilities were then measured using a Multiskan EX Microplate Photometer (Thermo Fisher Scientific) at 450 nm.
Colony-forming assay
TTFields were added to cells 6 h after sorafenib treatment to a last concentration of 3 µmol/L, and the cells were then maintained for 48 h. After 10–14 days, colonies were fixed with 100% methanol for 30 min and stained with 0.4% crystal violet (Sigma-Aldrich; Merck KGaA) according to the manufacturer’s instructions (8).
Flow cytometry
Floating and adherent cells were congregated and washed with phosphate-buffered saline (PBS). Cells were then centrifuged for 2 minutes at 1,500 rpm and resuspended in 1× Annexin-V binding buffer at a concentration of 1×105 cells/mL. The collected cells were stained with the Annexin V-APC/PI Apoptosis Kit (eBioscience, CA, USA) for 15 minutes at room temperature. The stained cells were analyzed by flow cytometry in accordance with the manufacturer’s protocols.
Cell death detection assay
Cells were treated, harvested, and stained with cell death kit detection reagent in accordance with the manufacturer’s protocols. Cell death was then measured using Multiskan EX (Thermo Fisher Scientific) at 450 nm.
Western blot analysis
Total proteins from cells were extracted in RIPA buffer (50 mM Tris-Cl, pH 7.4; 1% NP-40, 150 mM NaCl, and 1 mM EDTA) supplemented with protease inhibitors (1 mM PMSF, 1 µg/mL aprotinin, 1 µg/mL leupeptin, and 1 mM Na3VO4) and quantified using the Bradford method. Protein samples (30 µg) were separated by SDS/polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane used as described previously (13).
Fluorescent measurement of intracellular reactive oxygen species (ROS)
The fluorescent probe 2',7'-dichlorofluorescin diacetate (DCFH-DA) was utilized for the analysis of intracellular ROS. For fluorocytometrical estimation, cells were treated for 48 h with TTFields or sorafenib, or combination and were maintained for 30 minutes at room temperature with 10 µM DCFH-DA in 5 mL PBS. Fluorescence was measured with a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) in accordance with the manufacturer’s protocols (13).
Dual-luciferase reporter assay
With regard to the reporter gene assay, cells that were set in 24-well plates were transfected with 1 ng of the pRL-SV40 Renilla luciferase construct (as an internal control) for 24 h in addition to 200 ng of either pLucTK (the control plasmid) or pLucTKS3 (STAT3-responsive luciferase reporter plasmid) (24) prior to treatment for the condition that has been indicated. 36 hours after the treatment, preparation of the cell extracts was prepared and a measurement was carried out using dual-luciferase reporter assay system (Promega, Beijing, China) for the activity of luciferase, as has been ensured previously as well (25).
Statistical analysis
Statistical significance was determined using one-way analysis of variance (ANOVA). Values are the means ± standard deviation (SD) from three experiments. Differences were considered significant if the P value was less than 0.05 or 0.001. (*P<0.05; **P<0.01; ***P<0.001).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All in vitro experiments were approved by the Committee at Daegu Catholic University Medical Center.
Results
TTFields inhibit the proliferation of CRC cells in vitro
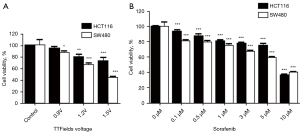
First, to study the sensitizing effects of combined treatment in CRC lines, we applied the indicated voltages of TTFields and doses of sorafenib to HCT116 and SW480 cells for 48 h to choose the optimal condition for TTFields (Figure 1A,1B). As shown in Figure 1, we showed that TTFields inhibited cell proliferation and cell viability in vitro in a voltage or dose-dependent manner in HCT116 and SW480 cells with nearly 20% viability inhibition observed at 0.9 V/cm and 3 µmol/L.
TTFields plus sorafenib inhibit the proliferation and clonogenicity of CRC cells in vitro
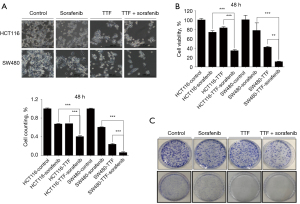
Next, HCT116 and SW480 cells were treated with sorafenib plus TTFields to evaluate combined sensitizing effect on CRC cells (Figure 2A,2B). Combined treatment with sorafenib and TTFields showed significantly further anti-cancer effects on the HCT116 and SW480 cells than either treatment alone, as estimated by using trypan blue cell-viability assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Figure 2A,2B). Furthermore, in the colony-forming assay, the clonogenicity of the cells was decreased after combined treatment with TTFields and sorafenib compared with those found after single treatments (Figure 2C). Our results proposed that sorafenib has a TTFields-sensitizing event on CRC cells in vitro.
TTFields plus sorafenib induce cell death in CRC cells
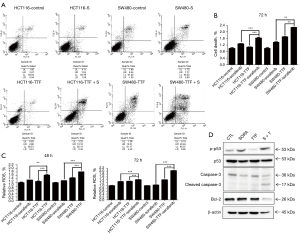
To investigate the mechanism by which combined TTFields and sorafenib therapy (herein after TTFields + sorafenib) induced the apoptosis of CRC cells were exposed to TTFields + sorafenib for 48 h, following which the population of Annexin V/propidium iodide-stained apoptotic cells was determined by flow cytometry and Cell death detection assay (Figure 3A,3B). According to the results of these experiments, it is noted that (Figure 3A) cell death in colon cancer cells resulted from the existence of TTFields + sorafenib; and (Figure 3B) a synergistic effect was produced as a result of the inclusion of sorafenib. Moreover, the association between the TTFields-induced apoptosis by sorafenib as well as ROS production was examined in this study. ROS production was induced to a greater extent by the combination of sorafenib and TTFields than by single treatment (Figure 3C), indicating that ROS produced by the combined treatment increases apoptosis compared with that of single treatment. Phosphorylation of p53 plays critical functions in controlling the biological activities of p53, and the phosphorylation of p53 at Ser-15 and Ser-20 is regulated in activating p53 (Figure 3D). Our result showed that increased levels of p-p53 (Ser-15) were enhanced synergistically after TTFields combined sorafenib treatment. TTFields + sorafenib treatment modified the expression of members of the BCL2 gene (26), which include protective proteins regulated in the mitochondrial apoptotic pathway. Correspondingly, the caspase-3 content decreased after TTFields + sorafenib treatment, indicating the content of cleaved protein (Figure 3D). Totally, these results show that apoptotic cell death by TTFields + sorafenib may happen through a caspase-dependent pathway.
TTFields plus sorafenib suppress AKT/STAT3 signaling in CRC cells
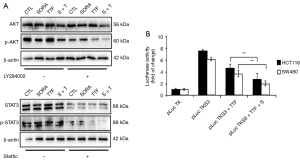
Western blot analyses were conducted for the verification of protein expression associated with apoptosis; this allowed to carry out an in-depth examination of the mechanisms that underpin the targeting of CRC growth by TTFields + sorafenib. Hence, a novel strategy for the targeting of CRC emerges from the combination therapy that inhibits the PI3K/AKT signaling pathway with the support of TTFields + sorafenib. In the context of the current study, the phosphorylation of AKT at the serine-473 site in CRC cells was visibly blockaded by the treatment using TTFields + sorafenib (Figure 4A) in addition to restraining the STAT3 (Figure 4A), which is notably activated by the phosphorylation of its tyrosine-705 residue in cancer (27). When treated with TTFields + sorafenib and LY294002 or Stattic, a selective AKT pathway inhibitor and STAT3 inhibitor, an additive inhibitory effect on AKT and STAT3 pathway was observed in HCT 116 cells. The protein levels of p-AKT or p-STAT3 in LY294002 or Stattic co-treated cells were much more reduced compared with that in inhibitor alone treated cells (Figure 4A). After its phosphorylation, STAT3 homodimerizes and translocates to the nucleus where it binds to specific STAT3 response elements in the promoter region of target genes to modulate transcription. With a specific STAT3-responsive luciferase reporter (24), we further studied through a luciferase reporter gene assay that TTFields + sorafenib suppressed STAT3 transactivation in CRC cells compared with that of single treatment (Figure 4B). Therefore, TTFields + sorafenib combination therapy inhibited AKT/STAT3 signaling in the CRC cells.
Discussion
In the last a few years, treatment paradigms with metastatic CRC have changed dramatically because agents like CYP-inhibitor abiraterone and second-generation AR antagonist enzalutamide have gained approvals. These new agents have been proven to extend the rate of survival through a distinct action mechanism (28). However, CRC cells are resistant to anti-cancer drug and are known to recur following chemotherapy, depending on the ratio of apoptotic cells to proliferating cells. Such resistances to existing cytotoxicic therapies curtail their success in most advanced cancer patients, especially in CRC. For this reason, further combined exploration of novel adjuvant agents as well as their potential therapeutic methodologies to treat CRC are important. Here, it has been observed that TTFields are new paradigm for CRC treatment, whereas sorafenib has also inhibited cellular growth and xenograft tumor growth of various metastatic CRC cells, wherein the inhibitory impacts of TTFields combined sorafenib on not only cell growth, but also colony formation was dose-dependent. As far as we know, this is the first study confirming that TTFields contribute CRC cells with cell death, which may compromise the therapeutic efficiency of preoperative radiotherapy.
Our new study has proven that TTFields combined sorafenib treatment majorly augments the cell apoptotic percent/cleavage of caspase-3 in CRC cells in a manner, thus suggesting that TTFields combined sorafenib causes the caspase-mediated cell apoptosis’ incidences across CRC cells. The PI3K pathway is activated in CRC cancers owing to deletions and mutations of PTEN and could also get involved in CRC progression, thus resulting in the activation and recruitment of AKT serine/threonine kinase (29). Afterward, it is responsible for regulating the proliferation, growth and survival of tumor cell. Thus, in activating or inhibiting PI3K/AKT signaling pathways is an important approach for treating CRC. This study further proved that TTFields combined sorafenib is capable of suppressing AKT’s phosphorylation on the site of serine473. In addition, inactivating AKT after TTFields combined sorafenib treatment has been found to inhabit the phosphorylation and transactivation of STAT3, thus suggesting that TTFields combined sorafenib is aimed at distinct signaling cascades regarding CRC treatment. STAT3 is activated by a tyrosine residue’s phosphorylation in the carboxy-terminal domain by a tyrosine kinase (Tyr705) which then homodimerizes and translocates into the nucleus where it is bound to target gene promoters’ distinct STAT3 response elements for regulating transcription (30). As per our findings, TTFields combined sorafenib do indeed modulate the expression of numerous anti-apoptotic and apoptotic gene, BCL-2, as all of which have been shown to be typical downstream target genes of STAT3. Hence, prior to the modulation of the expression BCL-2 gene in vitro, which is a crucial factor in the regulation of apoptosis associated with previously report on the cross-talk of STAT3 signaling modulating BCL-2 and PI3K/AKT expression in CRC progress, the activation of STAT3 and AKT was notably inhibited by TTFields + sorafenib (31). Collectively, our findings reveal a new mechanism for TTFields combined sorafenib that targets metastatic CRC tumor growth, wherein the inactivation of AKT/STAT3 by TTFields combined sorafenib treatment resulted in an altered expression of BCL-2 gene before inducing cell apoptosis and growth inhibition. For this reason, our results proved that TTFields combined sorafenib may be an efficacious promising clinical protocol to prevent the therapeutic use of metastatic CRC, that functions as a selective inhibitor of active AKT/STAT3 as well as a robust cell apoptosis inducer.
Acknowledgments
Funding: The work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu [2020].
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1853/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1853/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1853/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1853/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 2019;14:89-103. [Crossref] [PubMed]
- Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology 2008;134:1296-310. [Crossref] [PubMed]
- Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells. Sci Rep 2015;5:18046. [Crossref] [PubMed]
- Lee SX, Wong ET, Swanson KD. Mitosis interference of cancer cells by NovoTTF-100A causes decreased cellular viability. Cancer Res 2013;73:abstr 709.
- Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 2012;48:2192-202. [Crossref] [PubMed]
- Wong ET, Lok E, Swanson KD. An Evidence-Based Review of Alternating Electric Fields Therapy for Malignant Gliomas. Curr Treat Options Oncol 2015;16:40. [Crossref] [PubMed]
- Rehman AA, Elmore KB, Mattei TA. The effects of alternating electric fields in glioblastoma: current evidence on therapeutic mechanisms and clinical outcomes. Neurosurg Focus 2015;38:E14. [Crossref] [PubMed]
- Mrugala MM, Engelhard HH, Dinh Tran D, et al. Clinical practice experience with NovoTTF-100A™ system for glioblastoma: The Patient Registry Dataset (PRiDe). Semin Oncol 2014;41:S4-13. Erratum in: Semin Oncol 2015;42:e33-43. [Crossref] [PubMed]
- Pless M, Weinberg U. Tumor treating fields: concept, evidence and future. Expert Opin Investig Drugs 2011;20:1099-106. [Crossref] [PubMed]
- Toms SA, Tapinos N. Recent Advances in the Treatment of Gliomas - Comprehensive Brain Tumor Center. R I Med J (2013) 2017;100:43-6. [PubMed]
- Stupp R, Taillibert S, Kanner AA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015;314:2535-43. [Crossref] [PubMed]
- Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci 2013;1291:86-95. [Crossref] [PubMed]
- Helwick C, Goodman A. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): 2016 Guidelines. 2016. Available online: https://ascopost.com/issues/may-25-2016/nccn-clinical-practice-guidelines-in-oncology-nccn-guidelines-2016-guidelines/
- Jo Y, Kim EH, Sai S, et al. Functional Biological Activity of Sorafenib as a Tumor-Treating Field Sensitizer for Glioblastoma Therapy. Int J Mol Sci 2018;19:3684. [Crossref] [PubMed]
- Novocure. Novocure clinical pipeline. 2019. Available online: https://www.novocure.com/our-pipeline/
- Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets 2010;11:1000-17. [Crossref] [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [Crossref] [PubMed]
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293-300. [Crossref] [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [Crossref] [PubMed]
- Kacan T, Nayir E, Altun A, et al. Antitumor activity of sorafenib on colorectal cancer. Journal of Oncological Sciences 2016;2:53-7. [Crossref]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Lee YJ, Cho JM, Sai S, et al. 5-Fluorouracil as a Tumor-Treating Field-Sensitizer in Colon Cancer Therapy. Cancers (Basel) 2019;11:1999. [Crossref] [PubMed]
- Jeong H, Sung J, Oh S, et al. Inhibition of brain tumor cell proliferation by alternating electric fields. Appl Phys Lett 2014;105:203703. [Crossref]
- Turkson J, Bowman T, Garcia R, et al. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol 1998;18:2545-52. [Crossref] [PubMed]
- Wu K, Fan J, Zhang L, et al. PI3K/Akt to GSK3β/β-catenin signaling cascade coordinates cell colonization for bladder cancer bone metastasis through regulating ZEB1 transcription. Cell Signal 2012;24:2273-82. [Crossref] [PubMed]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008;9:47-59. [Crossref] [PubMed]
- Mora LB, Buettner R, Seigne J, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res 2002;62:6659-66. [PubMed]
- Gaya JM, Ahallal Y, Sanchez-Salas R, et al. Current, new and novel therapy for castration-resistant prostate cancer. Expert Rev Anticancer Ther 2013;13:819-27. [Crossref] [PubMed]
- Alexander SP, Fabbro D, Kelly E, et al. The concise guide to pharmacology 2017/18: Catalytic receptors. Br J Pharmacol 2017;174:S225-71. [Crossref] [PubMed]
- Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014;14:736-46. [Crossref] [PubMed]
- Abdulghani J, Allen JE, Dicker DT, et al. Sorafenib sensitizes solid tumors to Apo2L/TRAIL and Apo2L/TRAIL receptor agonist antibodies by the Jak2-Stat3-Mcl1 axis. PLoS One 2013;8:e75414. [Crossref] [PubMed]


