
Detection of circulating tumor cells and evaluation of epithelial-mesenchymal transition patterns of circulating tumor cells in ovarian cancer
Introduction
Ovarian cancer is known as the silent killer, with the highest mortality rate among female urogenital neoplasms. It is estimated that there will be approximately 21,410 new cases and 13,770 new deaths of ovarian cancer in the United States (1). The five-year survival rate for stage I (FIGO) is approximately 80%, but 75% patients have entered stage III or IV at the time of diagnosis, with less than 30% five-year survival rate (2). About 85% of patients still go through pelvic-abdominal recurrence and final death during treatment, even if the disease was completely relieved through the initial treatment (3). To improve the survival benefits, more effective methods should be developed and evaluated for early diagnosis, progression monitoring, drug resistance surveillance and recurrence detection during the disease management (4).
As an emerging minimal-invasive examination, liquid biopsy shows a great promise in precision oncology with its potential to dynamically reflect tumor progression and flexibly guide treatment in real time (5,6). Due to the intact cell structure and comprehensive information of both genes and proteins, circulating tumor cells (CTCs) have acquired much attention (7,8). Actually, CTCs are malignant single cells or cell clusters released into bloodstream from primary or metastatic lesion during tumor dissemination. The CTC clusters were suggested to possess 23- to 50-fold stronger metastatic ability, which are usually called the circulating tumor microemboli (CTM), consisting of more than 2 CTCs, platelets, fibroblasts and so on (9,10). Lots of works have been done to explore the diagnostic and prognostic value of CTC in ovarian cancer, especially in the evaluation of drug resistance and metastasis (11-15). A recent meta-analysis suggested that CTCs before the treatment implied worse survival outcomes and might be a potential biomarker in ovarian cancer (16).
However, faced with the pressure from the immune system and the fluid shear stress, the survival CTC are only 1–10 cells/mL of blood samples, while the number of white blood cells and red blood cells is 106/mL and 109/mL, respectively (17). Besides, the heterogeneity of epithelial-mesenchymal transition (EMT) status makes the application of the only epithelial marker-based biochemical technology into a dilemma (18,19). Due to the rarity and heterogeneity of CTC, the detection technology has always been a lasting challenge in the CTC clinical application. Recently, the CTC detection system using copper sulfide nanoparticles through photoacoustic detection can specifically identify CTC in ovarian cancer (20). Besides, a new photoacoustic flow cytometry platform realized vivo detection of CTC within 20 seconds in the bloodstream of melanoma patients, which also provided new thoughts about dynamic and efficient CTC detection (21).
During the process of tumor dissemination and metastasis, EMT is believed to be the initial step, with down-regulation of epithelial markers and up-regulation of mesenchymal markers of tumor cells. This phenotype alteration helps detached tumor cells including CTC to avoid the risk of anoikis and alleviate the tension from microenvironment, with stronger invasion ability. It was suggested that EMT status in CTCs might correlate with metastasis, recurrence and clinical stage of tumor (22-24). Ovarian cancer tumor cells are considered to go through partial or complete EMT, a potential target for the inhibition of metastasis (25,26). Vimentin expression is higher in solid metastases compared to primary carcinomas and effusions from ovarian cancer patients (27). Mesenchymal and intermediate mesenchymal ovarian cancer cells showed higher migration, adhesion and invasion capacities (28,29).
Nevertheless, only a few studies focused on the EMT patterns of CTCs in ovarian cancer. Previous study showed that EMT-like CTCs seem to be selected for platinum-based chemotherapy in ovarian cancer (30). A recent recurrence risk stratification showed the considerable potential of the mesenchymal–CTCs to predict recurrence in ovarian cancer, emphasizing the prognostic value of CTCs undergoing EMT (31). Herein, for exploration of detection method with higher positive rate of CTCs in ovarian cancer, we introduced a novel method combining the microfiltration and morphological analysis for the CTC detection. Moreover, for comprehensive understanding the invasiveness of disseminated tumor cells in ovarian cancer, EMT markers, epithelial marker (Cytokeratin) and mesenchymal marker (Vimentin), were detected in CTCs/CTM in blood and exfoliated tumor cells in ascites. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-529/rc).
Methods
Patients and samples
The 22 subjects with newly diagnosed or relapsed ovarian cancer planning to receive surgery or chemotherapy in the Obstetrics and Gynecology Hospital of Fudan University were included in the study. The collection of the clinicopathological characteristics was performed at the time of enrollment before surgery or chemotherapy, including age, serum CA125 level and so on. About 8 mL of peripheral blood were obtained from 22 patients before the surgery and 10 mL of ascites were obtained from 22 patients during surgery. The Cell Save Preservative Tube (Veridex LLC, Raritan, NJ, USA) were used to store and transport samples from patients at 4 ℃, processed for further analysis within 24 hours.
CTC enrichment and isolation
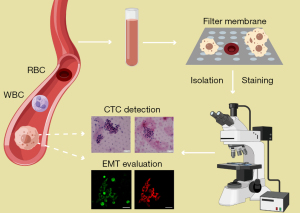
CTCs were enriched from blood samples by the microfiltration method with the CTCBIOPSY® system (Wuhan YZY Medical Science and Technology Co., Ltd., Wuhan, China) according to the manual instructions (Figure 1). The system is based on the ISET platform (isolation by size of tumor cells), with higher CTC positivity than the CellSearch system (32,33). Before loading, 5 mL of the samples were fixed at the room temperature for 10 minutes in the 15-mL centrifuge tubes, containing 3 mL 0.9% physiological saline and 200 µL 0.2% paraformaldehyde (PFA). Then the quality assessment and equipment cleaning of the filtration system were carried out with 75% alcohol and 0.9% physiological saline. The diluted mixture was loaded into wells and then filtered through porous polycarbonate membranes by the positive pressures. The membranes with 8 µm cylindrical pores of the device retain larger and less deformable CTCs while allowing smaller blood cells to pass through, regardless the cell surface protein expression.
CTC detection and characterization
The detection of the CTCs was performed with morphological identification (Figure 1). The clusters of at least 3 CTCs were defined as CTM. The membrane with captured CTCs and CTM was taken out and placed on slides. The cells were fixed with 4% PFA for 30 minutes, followed by washing, permeabilization and blocking for further staining. As crucial makers of EMT, the epithelial marker cytokeratin and the mesenchymal marker vimentin were chosen to evaluate EMT in CTCs by immunofluorescence (23,34-36). Antibodies used were including FITC Anti-Cytokeratin antibody (ab52459, abcam) and Alexa Fluor® 647 Anti-Vimentin antibody (ab195878, abcam). After fluorescence microscopy, the cells on the membrane were further stained with hematoxylin and eosin (H&E) for cytomorphological analysis. The isolated cells were microscopically examined by three experienced pathologists to avoid false-positive results. Only cells that appear as large cells with deep stained nuclei and/or atypia, which are features of tumor cells under the microscope, were identified as CTCs. The criteria for the confirmation of CTCs are shown in Table S1 (37).
Statistical analysis
The SPSS 19.0 (SPSS Inc., USA) software was used for statistical analysis. The cell counting was carried out using mean ± standard deviation (mean ± SD). Group differences were analyzed by Fisher’s exact test and chi-square test. P value <0.05 was considered significant.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Obstetrics and Gynecology Hospital of Fudan University (No. 2016-48), and written informed consent was taken from all the patients.
Results
Patient characteristics
A total of 22 women aged 36–71 years were enrolled in the study, with pathologically confirmed ovarian cancer (FIGO stage II-IV). Among all the patients, 19 (86.4%) were sensitive to platinum and 5 (22.7%) had pathologic lymphatic metastasis. The serum carbohydrate antigen 125 (CA125) level of most patients were at least 100 IU/L when enrolled in the study. The clinicopathological details of CTC-positive patients and CTC-negative patients were shown in Table 1.
Table 1
| Parameters | Details | No. (n=22) | No. of CTC/CTM | |
|---|---|---|---|---|
| Positive (n=9) | Negative (n=13) | |||
| Age, years | ≤40 | 2 | 1 | 1 |
| >40 | 20 | 8 | 12 | |
| Median | 54.5 [36–71] | |||
| Stage (FIGO) | II | 1 | 0 | 1 |
| III | 18 | 9 | 9 | |
| IV | 2 | 0 | 2 | |
| Unknown | 1 | 0 | 1 | |
| Pathologic types | Serous carcinoma | 17 | 7 | 10 |
| Clear-cell carcinoma | 2 | 1 | 1 | |
| Endometrioid carcinoma | 1 | 0 | 1 | |
| Mucinous carcinoma | 1 | 0 | 1 | |
| Krukenburg tumor | 1 | 1 | 0 | |
| Platinum response | Sensitive | 19 | 8 | 11 |
| Resistant | 3 | 1 | 2 | |
| Lymphatic metastasis | Positive† | 5 | 2 | 3 |
| Negative‡ | 17 | 7 | 10 | |
| Serum CA125 levels (IU/L) | >1,000 | 8 | 3 | 5 |
| 100–1,000 | 7 | 3 | 4 | |
| <100 | 4 | 2 | 2 | |
| Unknown | 3 | 1 | 2 | |
†, systematic lymphadenectomy or surgery was performed. ‡, no enlarged lymph nodes were detected in the 17 patients through intraoperative exploration or imaging studies without systematic lymphadenectomy or surgery. CTC, circulating tumor cell; CTM, circulating tumor microemboli.
Isolation and identification of CTC in ovarian cancer
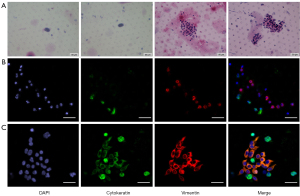
The microfiltration combined with cytomorphological analysis was applied into CTC detection. In total, 40.9% (9/22) of the patients had ≥1 CTCs or CTM; this rate was relatively higher than the CTCs/CTM detection rate reported in previous studies using immunoaffinity-based methods (5). The single CTC number varied from 1 to 8, with CTM number ranging from 4 to 30. Clinical details and CTC numbers of the 9 patients are shown in Table 2. The H&E staining results of CTCs/CTM are relatively shown in Figure 2. According to the criteria, the CTCs/CTM of ovarian cancer patients exhibited features such as large cells with deep stained nuclei or nuclear atypia under the microscope.
Table 2
| CTCs number | CTM number | Cytokeratin positive | Clinicopathological details | |||||
|---|---|---|---|---|---|---|---|---|
| Age | Stage (FIGO) | Pathologic types | Platinum response | Lymphatic metastasis | Serum CA125 levels (IU/L) | |||
| 0 | 30 | 4 | 36 | IIIC | Krukenburg tumor | Sensitive | Negative‡ | 182.5 |
| 1 | 0 | 0 | 44 | IIIB | Clear-cell carcinoma | Sensitive | Negative‡ | 40.21 |
| 0 | 1 | 48 | IIIC | Serous carcinoma | Sensitive | Negative‡ | 982.6 | |
| 0 | 1 | 70 | IIIC | Serous carcinoma | Sensitive | Negative‡ | 48.3 | |
| 6 | 0 | 52 | IIIC | Serous carcinoma | Sensitive | Positive† | >1,000 | |
| 2 | 0 | 1 | 47 | IIIC | Serous carcinoma | Resistant | Negative‡ | >1,000 |
| 3 | 0 | 0 | 46 | IIIA | Serous carcinoma | Sensitive | Positive† | >1,000 |
| 4 | 1 | 0 | 58 | IIIC | Serous carcinoma | Sensitive | Negative‡ | 745.9 |
| 8 | 0 | 0 | 71 | IIIC | Serous carcinoma | Sensitive | Negative‡ | 171.5 |
†, systematic lymphadenectomy or surgery was performed; ‡, no enlarged lymph nodes were detected through intraoperative exploration or imaging studies without systematic lymphadenectomy or surgery. CTCs, circulating tumor cells; CTM, circulating tumor microemboli.
EMT pattern of blood CTCs and ascites disseminated tumor cells in ovarian cancer
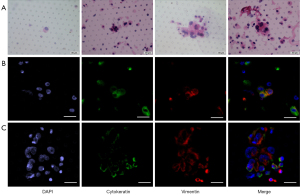
To evaluate the EMT pattern of disseminated tumor cells in blood, vimentin and cytokeratin were immunostained and evaluated in isolated CTCs/CTM in ovarian cancer (Figure 2). It was found that vimentin was widely expressed in all samples with detected CTCs/CTM, while cytokeratin was positive in only 44.4% of samples. To evaluate the invasive capacity of disseminated tumor cells in ascites, EMT was also evaluated using cytokeratin and vimentin (Figure 3). Similarly, vimentin can be detected in all exfoliated tumor cells in ascites, but cytokeratin is lowly expressed or not expressed. The upregulation of the mesenchymal marker vimentin along with the downregulation of the epithelial marker cytokeratin in CTCs/CTM and tumor cells in ascites indicated that CTCs/CTM disseminated tumor cells in both blood and ascites might have higher invasiveness, with partial or complete EMT.
Preliminary analysis of correlation between CTC and clinical parameters
According to the CTCs/CTM detection, CTC counts were stratified as negative group (no detectable CTC) or positive group (at least 1 detectable CTC or CTM) for analysis of the correlation with clinicopathological variables. Through the subgroup analysis, no significant correlation was found between CTC positivity and characteristics including age, pathologic stage, pathologic type, lymphatic metastasis, platinum response or serum CA125 level. The relationship between the clinicopathological features of patients and the number of CTCs was not analyzed because of the small sample size.
Discussion
Given the potential value of CTC in oncology, the technology of CTC capture and detection increasingly appeared these years, mainly based on the physical properties such as size, density or deformability and biological properties like surface antigens (38,39). The only one FDA-approved platform, the well-known CellSearch assay, was based on epithelial proteins including epithelial cell adhesion molecule (EpCAM) and cytokeratin. This technology was commonly used in ovarian cancer (40-42). Due to the phenotypic alteration caused by EMT process, the CTCs/CTM with low EpCAM or cytokeratin expression can be missed through the method only using epithelia markers, causing false negatives and inaccurate evaluation of clinical value (43,44). Some studies combined both epithelial and mesenchymal markers to improve the CTC detection rate of ovarian cancer, whereas the combination caused some false positives (45). Recently, methods based on physical properties brought technological progress in CTC studies. The size-based MetaCell platform and some microfluidic devices were applied in CTC capture in ovarian cancer (46,47). These technologies obtained higher CTC positive rate than the immunoaffinity-based methods, avoiding the potential heterogeneity of CTC capture rate caused by EMT process.
Considering the positivity and EMT, our study focused on effective size-based method for CTC studies in ovarian cancer. Among all the physical methods, the ISET filtration technology showed a satisfactory CTC positive rate in gastric cancer (59.32%), non-small cell lung cancer (88%), pancreatic cancer (93%) (37,48,49). Herein, the CTCBIOPSY® platform based on the ISET technology was first applied in CTC detection in ovarian cancer. To avoid the false positives caused by clusters of the blood cells, the cytomorphological verification was combined with ISET. The CTCs/CTM positivity rate in ovarian cancer based on ISET technology is lower than other tumors partly because of the differential metastasis. However, the rate is still higher than most previous studies on ovarian cancer (13,50,51). The result also shows the true positive rate of CTC in ovarian cancer was indeed higher than majority of earlier CTC studies. Besides, the method in our study might provide an alternative way of CTC detection for further studies of CTCs/CTM, using microfiltration combining with cytomorphological analysis. Nevertheless, compared with satisfactory positivity of the tapered slit filter and nanoroughened microfluidic platform, our results should be verified with larger samples (12,52). Hence, more robust combination of enrichment methods and detection technology based on physical or biological traits should be further performed in the CTC evaluation.
To some extent, CTC can help us understand the metastasis mechanism and the genetic information of ovarian cancer, which can be targeted for early diagnosis and treatment. The EMT evaluation of the CTCs/CTM in blood and the tumor cells in ascites in ovarian cancer really contributes to better understanding of hematogenous and peritoneal metastasis of ovarian cancer. In this study, the EMT pattern of disseminated tumor cells was evaluated for further exploration through the detection of vimentin and cytokeratin in 22 ovarian cancer patients. The mesenchymal marker vimentin was expressed in all CTCs/CTM and all tumor cells in ascites, yet epithelial marker cytokeratin in less than half of these cells. This alteration suggests that the detached ovarian cancer cells might have undergone EMT to improve invasion ability during the process of dissemination. Besides, the results also explain the heterogeneous CTC positivity using the CellSearch in ovarian cancer, varying from 14% to 60% in multiple works, as reviewed previously (5). However, only vimentin was used to detect EMT of detached tumor cells in this study. For accurate evaluation of the EMT phenotype of CTCs, E cadherin, N cadherin and transcription factors of EMT should be examined in the future works. As a complex of multiple cells, CTM was suggested to be more aggressive than single CTCs, although it was not elucidated clearly (53). Recent single-cell analysis showed the disseminated tumor cell clusters in the ovarian cancer ascites acquired invasive property via activation of EMT through upregulation of the transcription factor of EMT (28). However, we did not compare the EMT status between CTCs and CTM due to the small sample size.
The existence and heterogeneity of EMT process is believed to participate in cancer invasiveness and dissemination in the early stage of metastasis, contributing to the generation of CTCs/CTM. As reported previously in the correlation analysis, the expression of EMT markers in CTC might be associated with clinical variables such as tumor size, stages and relapse condition (23,54). EMT process can help tumor clusters in ascites possess additional metastasis capacity and drug resistance in ovarian cancer (28). Hence, the inhibition of EMT signaling pathways is expected to block the potential micrometastasis in the initial stage of metastasis, one of the main problems in ovarian cancer. The TGFβ, WNTs, NOTCH and other signaling pathways induce the expression of EMT transcription factors including the zinc finger E-box binding homeobox 2 ZEB, the zinc finger transcription factor SNAIL and the basic helix-loop-helix factor TWIST (55). Inhibitors of stimuli, extracellular mediators and intracellular signaling pathways in EMT have been developed and applicated in cancer treatment (56). Appropriate evaluation of EMT in CTCs/CTM builds the foundation for inhibitors application in ovarian cancer. To understand the metastasis mechanism, we will also focus on the transcriptional and genomic landscape of disseminated ovarian cancer cells in the future works.
There are some limitations in our study. The sample size and heterogeneous histology of our samples are the main deficiencies. The relatively small sample size makes the relationship between clinicopathological features and CTC positivity in ovarian cancer not conclusive. The comparative analysis of pre- and post-treatment was not carried out in this study. In addition, after cytomorphology and immunofluorescence detection, there were few CTCs remaining for further sequencing or biological property analysis in the next work. In all, further analyzation and verification need to be done by larger sample size.
Conclusions
In this study, we detected CTCs/CTM in ovarian cancer patients using microfiltration combined with cytomorphological analysis, which reduced the risk due to false negatives for CTCs with EMT and false positives due to a cluster of white blood cells and showed a higher detection rate. We also confirmed that the CTCs/CTM and detached tumor cells in ascites exhibited complete or partial EMT, which might reflect the dissemination and micrometastasis of ovarian cancer. Clinical values of CTCs/CTM need to be validated with larger patient cohorts.
Acknowledgments
Funding: This work was funded by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-529/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-529/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-529/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-529/coif). CJX reports funding made to the Obstetrics and Gynecology Hospital of Fudan University from the Shanghai Medical Center of Key Programs for Female Reproductive Diseases (grant No. 2017ZZ01016). XYZ reports funding made to the Obstetrics and Gynecology Hospital of Fudan University from the National Natural Science Foundation of China (grant No. 82172747). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin 2019;69:280-304. [Crossref] [PubMed]
- Corrado G, Salutari V, Palluzzi E, et al. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther 2017;17:1147-58. [Crossref] [PubMed]
- Lheureux S, Gourley C, Vergote I, et al. Epithelial ovarian cancer. Lancet 2019;393:1240-53. [Crossref] [PubMed]
- Asante DB, Calapre L, Ziman M, et al. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time? Cancer Lett 2020;468:59-71. [Crossref] [PubMed]
- Giannopoulou L, Lianidou ES. Liquid biopsy in ovarian cancer. Adv Clin Chem 2020;97:13-71. [Crossref] [PubMed]
- Magbanua MJM, Hendrix LH, Hyslop T, et al. Serial Analysis of Circulating Tumor Cells in Metastatic Breast Cancer Receiving First-Line Chemotherapy. J Natl Cancer Inst 2021;113:443-52. [Crossref] [PubMed]
- Ahn JC, Teng PC, Chen PJ, et al. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021;73:422-36. [Crossref] [PubMed]
- Dive C, Brady G. SnapShot: Circulating Tumor Cells. Cell 2017;168:742-742.e1. [Crossref] [PubMed]
- Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110-22. [Crossref] [PubMed]
- Zhang X, Li H, Yu X, et al. Analysis of Circulating Tumor Cells in Ovarian Cancer and Their Clinical Value as a Biomarker. Cell Physiol Biochem 2018;48:1983-94. [Crossref] [PubMed]
- Lee M, Kim EJ, Cho Y, et al. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol Oncol 2017;145:361-5. [Crossref] [PubMed]
- Obermayr E, Bednarz-Knoll N, Orsetti B, et al. Circulating tumor cells: potential markers of minimal residual disease in ovarian cancer? a study of the OVCAD consortium. Oncotarget 2017;8:106415-28. [Crossref] [PubMed]
- Pearl ML, Dong H, Tulley S, et al. Treatment monitoring of patients with epithelial ovarian cancer using invasive circulating tumor cells (iCTCs). Gynecol Oncol 2015;137:229-38. [Crossref] [PubMed]
- Dong H, Tulley S, Zhao Q, et al. The propensity of invasive circulating tumor cells (iCTCs) in metastatic progression and therapeutic responsiveness. Cancer Med 2019;8:3864-74. [Crossref] [PubMed]
- Huang C, Lin X, He J, et al. Enrichment and detection method for the prognostic value of circulating tumor cells in ovarian cancer: A meta-analysis. Gynecol Oncol 2021;161:613-20. [Crossref] [PubMed]
- Alvarez Cubero MJ, Lorente JA, Robles-Fernandez I, et al. Circulating Tumor Cells: Markers and Methodologies for Enrichment and Detection. Methods Mol Biol 2017;1634:283-303. [Crossref] [PubMed]
- Liu H, Zhang X, Li J, et al. The biological and clinical importance of epithelial-mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol 2015;141:189-201. [Crossref] [PubMed]
- Wu S, Liu S, Liu Z, et al. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One 2015;10:e0123976. [Crossref] [PubMed]
- Lusk JF, Miranda C, Howell M, et al. Photoacoustic Flow System for the Detection of Ovarian Circulating Tumor Cells Utilizing Copper Sulfide Nanoparticles. ACS Biomater Sci Eng 2019;5:1553-60. [Crossref] [PubMed]
- Galanzha EI, Menyaev YA, Yadem AC, et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci Transl Med 2019;11:eaat5857. [Crossref] [PubMed]
- Qi LN, Xiang BD, Wu FX, et al. Circulating Tumor Cells Undergoing EMT Provide a Metric for Diagnosis and Prognosis of Patients with Hepatocellular Carcinoma. Cancer Res 2018;78:4731-44. [Crossref] [PubMed]
- Li YM, Xu SC, Li J, et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis 2013;4:e831. [Crossref] [PubMed]
- Chen J, Cao SW, Cai Z, et al. Epithelial-mesenchymal transition phenotypes of circulating tumor cells correlate with the clinical stages and cancer metastasis in hepatocellular carcinoma patients. Cancer Biomark 2017;20:487-98. [Crossref] [PubMed]
- Dong P, Xiong Y, Watari H, et al. MiR-137 and miR-34a directly target Snail and inhibit EMT, invasion and sphere-forming ability of ovarian cancer cells. J Exp Clin Cancer Res 2016;35:132. [Crossref] [PubMed]
- Yildirim N, Kocal GC, Isik Z, et al. Ubiquitin-Proteasome Axis, Especially Ubiquitin-Specific Protease-17 (USP17) Gene Family, is a Potential Target for Epithelial-Mesenchymal Transition in High-Grade Serous Ovarian Cancer. Reprod Sci 2019;26:794-805. [Crossref] [PubMed]
- Elloul S, Vaksman O, Stavnes HT, et al. Mesenchymal-to-epithelial transition determinants as characteristics of ovarian carcinoma effusions. Clin Exp Metastasis 2010;27:161-72. [Crossref] [PubMed]
- Kan T, Wang W, Ip PP, et al. Single-cell EMT-related transcriptional analysis revealed intra-cluster heterogeneity of tumor cell clusters in epithelial ovarian cancer ascites. Oncogene 2020;39:4227-40. [Crossref] [PubMed]
- Rosso M, Majem B, Devis L, et al. E-cadherin: A determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PLoS One 2017;12:e0184439. [Crossref] [PubMed]
- Chebouti I, Kasimir-Bauer S, Buderath P, et al. EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget 2017;8:48820-31. [Crossref] [PubMed]
- Yang J, Ma J, Jin Y, et al. Development and validation for prognostic nomogram of epithelial ovarian cancer recurrence based on circulating tumor cells and epithelial-mesenchymal transition. Sci Rep 2021;11:6540. [Crossref] [PubMed]
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15. [Crossref] [PubMed]
- Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 2000;156:57-63. [Crossref] [PubMed]
- Liu PF, Kang BH, Wu YM, et al. Vimentin is a potential prognostic factor for tongue squamous cell carcinoma among five epithelial-mesenchymal transition-related proteins. PLoS One 2017;12:e0178581. [Crossref] [PubMed]
- Niknami Z, Muhammadnejad A, Ebrahimi A, et al. Significance of E-cadherin and Vimentin as epithelial-mesenchymal transition markers in colorectal carcinoma prognosis. EXCLI J 2020;19:917-26. [PubMed]
- Yi BR, Kim TH, Kim YS, et al. Alteration of epithelial-mesenchymal transition markers in human normal ovaries and neoplastic ovarian cancers. Int J Oncol 2015;46:272-80. [Crossref] [PubMed]
- Ning D, Cui K, Liu M, et al. Comparison of CellSearch and Circulating Tumor Cells (CTC)-Biopsy Systems in Detecting Peripheral Blood Circulating Tumor Cells in Patients with Gastric Cancer. Med Sci Monit 2021;27:e926565. [Crossref] [PubMed]
- Bankó P, Lee SY, Nagygyörgy V, et al. Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol 2019;12:48. [Crossref] [PubMed]
- Habli Z, AlChamaa W, Saab R, et al. Circulating Tumor Cell Detection Technologies and Clinical Utility: Challenges and Opportunities. Cancers (Basel) 2020;12:1930. [Crossref] [PubMed]
- Liu JF, Kindelberger D, Doyle C, et al. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol Oncol 2013;131:352-6. [Crossref] [PubMed]
- Behbakht K, Sill MW, Darcy KM, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol 2011;123:19-26. [Crossref] [PubMed]
- Poveda A, Kaye SB, McCormack R, et al. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol 2011;122:567-72. [Crossref] [PubMed]
- de Wit S, van Dalum G, Lenferink AT, et al. The detection of EpCAM(+) and EpCAM(-) circulating tumor cells. Sci Rep 2015;5:12270. [Crossref] [PubMed]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. [Crossref] [PubMed]
- Po JW, Roohullah A, Lynch D, et al. Improved ovarian cancer EMT-CTC isolation by immunomagnetic targeting of epithelial EpCAM and mesenchymal N-cadherin. J Circ Biomark 2018;7:1849454418782617. [Crossref] [PubMed]
- Guo YX, Neoh KH, Chang XH, et al. Diagnostic value of HE4+ circulating tumor cells in patients with suspicious ovarian cancer. Oncotarget 2018;9:7522-33. [Crossref] [PubMed]
- Obermayr E, Maritschnegg E, Agreiter C, et al. Efficient leukocyte depletion by a novel microfluidic platform enables the molecular detection and characterization of circulating tumor cells. Oncotarget 2017;9:812-23. [Crossref] [PubMed]
- Tamminga M, Andree KC, Hiltermann TJN, et al. Detection of Circulating Tumor Cells in the Diagnostic Leukapheresis Product of Non-Small-Cell Lung Cancer Patients Comparing CellSearch® and ISET. Cancers (Basel) 2020;12:896. [Crossref] [PubMed]
- Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer 2012;106:508-16. [Crossref] [PubMed]
- Pearl ML, Zhao Q, Yang J, et al. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol Oncol 2014;134:581-90. [Crossref] [PubMed]
- Kuhlmann JD, Wimberger P, Bankfalvi A, et al. ERCC1-positive circulating tumor cells in the blood of ovarian cancer patients as a predictive biomarker for platinum resistance. Clin Chem 2014;60:1282-9. [Crossref] [PubMed]
- Kim M, Suh DH, Choi JY, et al. Post-debulking circulating tumor cell as a poor prognostic marker in advanced stage ovarian cancer: A prospective observational study. Medicine (Baltimore) 2019;98:e15354. [Crossref] [PubMed]
- Umer M, Vaidyanathan R, Nguyen NT, et al. Circulating tumor microemboli: Progress in molecular understanding and enrichment technologies. Biotechnol Adv 2018;36:1367-89. [Crossref] [PubMed]
- Tada H, Takahashi H, Ida S, et al. Epithelial-Mesenchymal Transition Status of Circulating Tumor Cells Is Associated With Tumor Relapse in Head and Neck Squamous Cell Carcinoma. Anticancer Res 2020;40:3559-64. [Crossref] [PubMed]
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20:69-84. [Crossref] [PubMed]
- Marcucci F, Stassi G, De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov 2016;15:311-25. [Crossref] [PubMed]


