
Durable clinical response to ALK tyrosine kinase inhibitors in ALK-rearranged non-small cell lung cancer: a case report
Introduction
Anaplastic lymphoma kinase (ALK) rearrangement is found in 5–6% of all non-small cell lung cancers (NSCLCs) (1). It is a key oncogenic driver resulting in AKI protein overexpression and activation of its kinase domain. The first-generation ALK-tyrosine kinase inhibitor (TKI) crizotinib is the standard treatment for ALK rearrangement NSCLC (2). However, almost all patients treated with crizotinib develop resistance, mediated mainly by acquired secondary ALK resistance mutations (3).
The second-generation TKIs, such as brigatinib, are effective in crizotinib-resistant patients with secondary mutation (4). Lorlatinib is a reversible third-generation ALK inhibitor for the treatment of ALK-positive NSCLC; and it can overcome multiple ALK TKIs resistance mutations and shows promising activity against brain metastasis (5).
Investigating how specific acquired mutations predict sensitivity to ALK kinase inhibitors is urgent. Here, we discuss ALK E1129V, F1174C, F1174L, F1174V, I1171T, and G1269A mutations simultaneously emerging during treatment with crizotinib for an NSCLC patient harboring an EML4-ALK rearrangement, who then responded to brigatinib therapy. Five ALK mutations were not detected at brigatinib progression, and L1196M was detected at lorlatinib progression. The patient achieved a durable clinical response to ALK TKIs (crizotinib, brigatinib, and lorlatinib). We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2838/rc).
Case presentation
A 61-year-old Chinese woman without a smoking history was diagnosed with stage IIIB lung adenocarcinoma (Figure 1A and 1B) in a local hospital in July 2017. The patient underwent a fine-needle biopsy of the left lower lobe. No rearrangement or amplification of ALK, ROS, and MET was found by fluorescence in situ hybridization (FISH) and no EGFR and KRAS mutation was found in exon 18–21 using the droplet digital PCR (ddPCR) system. The patient was initially treated with two cycles of chemotherapy regimens of pemetrexed and cis-platinum, then changed to four cycles of pemetrexed and carboplatin because of adverse effects. Stable disease (SD) was achieved after three months; therefore, the patient underwent stereotactic body radiation therapy (SBRT) in the lung lesion of the left lower lobe in January 2018. The prescription dose of SBRT was 95% PGTV 50Gy/50F. Her computed tomography (CT) revealed thoracic spinal metastasis in May 2018, and palliative radiotherapy against T3-T6 and T8-T10 was used to relieve pain in the lower back and the right hip. The therapeutic course (Figure 1C), and radiological examinations (Figure 1D-1G) are summarized in Figure 1.
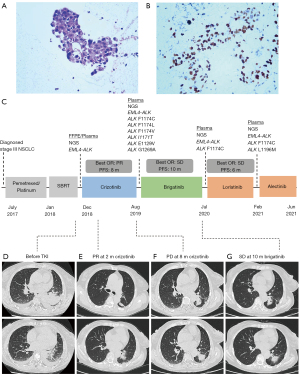
The patient was referred to our hospital for further treatment in November 2018. Her chest CT scanning showed an enlarged primary tumor mass lesion (50 mm × 44 mm) in the left lower lobe, multiple lymph nodes and pleural effusions (Figure 1D), and liver metastasis (19.4 mm × 13.6 mm), indicating tumor progression. Bronchoscopy and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed to obtain the biopsies of tissue from the left lower lobe and lymph nodes. To explore cancer-related genetic alterations, DNA-based next-generation sequencing (NGS) was performed using a targeted panel consisting of 168 cancer-related genes (Lung Plasma, Burning Rock, Guangzhou, China) with lung biopsy specimens and whole blood cells. EML4-ALK (E13:A20, indicating the fusion variant 1 between EML4 exon 13 and ALK exon 20) rearrangement was identified in both tissue and plasma circulating tumor DNA (ctDNA). The allelic fraction (AF) was 11.09%. No other ALK mutation was detected (Figure 2A). Subsequently, the patient initiated crizotinib (250 mg, bid) treatment in December 2018. The patient achieved a partial response (PR) two months later with shrunken primary (39.3 mm × 34.8 mm) and metastatic tumors (Figure 1E), and a significant reduction in the blood tumor markers, particularly carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) (Figure 2B). The progression-free survival (PFS) was 8 months. Follow-up CT imaging showed three low-density masses in the left lung in August 2019 (Figure 1F). Magnetic resonance imaging (MRI) showed multiple small contrast-enhancing lesions, suggesting brain metastasis. Crizotinib was discontinued.

To identify the mechanism underlying resistance to crizotinib, ctDNA was analyzed using NGS (Lung Plasma, Burning Rock, Guangzhou, China). In addition to EML4-ALK rearrangement, six ALK point mutations were detected, including E1129V, F1174C, F1174L, F1174V, I1171T, and G1269A (Figure 2A). Except for E1129V, other mutations are reported as a resistance mechanism of ALK inhibitor crizotinib but are sensitive to the second-generation ALK inhibitor brigatinib (6). Therefore, brigatinib was administered in September 2019. She experienced an overall improvement in her clinical symptoms. The levels of CA19-9 and CEA were also observed to be decreased after brigatinib treatment, especially the CA19-9 level was close to the normal range during brigatinib treatment (Figure 2B). The patient obtained SD with 10 months of PFS. A follow-up CT scan showed the primary lung tumors were stable (Figure 1G).
However, the patient tended to have worse ability to perform activities and higher levels of chest pain and cough, and plasma was sent for NGS in July 2020. ctDNA analysis showed the retention of EML4-ALK and ALK F1174C, but the other five mutations were not detected without the emergence of new mutations (Figure 2A). The abundance of EML4-ALK and ALK F1174C (AF 51.81% and 65.67%) was significantly higher than before brigatinib treatment, indicating disease progression. So brigatinib was discontinued. Subsequently, the patient was switched to lorlatinib and her general condition improved during that time with a PFS of 6 months. In February 2021, the patient experienced dyspnea, anorexia, nausea, worsening chest pain, and appeared cachectic. Her performance score was 3. ctDNA analysis by NGS detected the emergence of ALK L1196M (AF 2.72%) and retention of EML4-ALK (AF 57.14%) and ALK F1174C (AF 70.72%) (Figure 2A). Alectinib was initiated with the patient's consent but she experienced vomiting and severe nausea, which may lead to the oral medication not being effective. The patient's general condition worsened, and she passed away in a local hospital with the an overall survival (OS) of about four years.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We herein report a patient who achieved a durable clinical response to the sequential use of ALK TKIs, crizotinib, brigatinib, and lorlatinib and underwent multiple repeat liquid biopsies throughout the course of the disease. A female patient was presented with stage IIIB disease and no common driver mutations were detected with FISH and ddPCR. So she was initially treated with chemotherapy and radiation therapy with no good response. She then developed stage IV disease with lymph node and liver metastasis. With DNA-based NGS, EML4-ALK variant 1 was detected both in tissue and plasma. The patient achieved a rapid and excellent clinical response to crizotinib with relief of all symptoms. ALK E1129V, F1174C, F1174L, F1174V, I1171T, and G1269A mutations emerged at progression with brain metastasis then responded to brigatinib therapy. With sequential ctDNA analysis by NGS, lorlatinib was used based on gene mutations. The OS was 4 years.
Five FDA-approved ALK TKIs include crizotinib, brigatinib, lorlatinib, ceritinib, and alectinib. ALK resistance mutations that emerge during treatment significantly limit their effectiveness (7). In our case, the best response to crizotinib treatment was PR, and the PFS was 8 months. Secondary mutations in the tyrosine kinase domain of ALK confer resistance to crizotinib, including ALK L1196M, G1269A, C1156Y, G1202R, I1171T/N/S, S1206C/Y, E1210K, L1152P/R, V11180L, I1151T, G1128A, and F1174V (6). The number of ALK resistance mutations increases with each successive generation of ALK TKI (8). In our case, we simultaneously detected six mutations from ctDNA after only TKI treatment once, which is rare. Patient with ALK E1129V, F1174C, F1174L, F1174V, I1171T, and G1269A responded to brigatinib lasting 10 months and brigatinib was discontinued when her general condition worsened, at which time liquid biopsy detected the accumulation of EML4-ALK variant 1 and ALK F1174C. ALK F1174C is reported to be resistant to crizotinib but sensitive to brigatinib (6,9). However, we noticed the ALK F1174C might be the resistance mutation of brigatinib. ALK F1174C was also reported to be sensitive to lorlatinib and alectinib (6). Patients harboring ALK F1174X mutation (including known and unknown F1174 missense mutations, like F1174 C/I/L/M/S/V etc.) show a good response to lorlatinib in a phase II study of advanced ALK-positive NSCLC patients (7). On the basis of this finding, the patient was switched to lorlatinib and achieved SD for 6 months. When she relapsed on lorlatinib, ALK L1196M was detected with the stepwise accumulation of ALK resistance mutations EML4-ALK variant 1 and ALK F1174C. ALK L1196M was the first reported resistance mutation (10), generating resistance to crizotinib. We detected ALK L1196M after resistance to lorlatinib. An in vitro study showed that compound ALK mutations F1174C and L1196M could cause resistance to lorlatinib (11). The patient’s clinical condition was poor upon lorlatinib progression, and treatment of alectinib was not effective due to her vomiting and severe nausea.
With the development of NGS technology, liquid biopsy has now been used to monitor the genetic alterations of ctDNA. It plays a significant role in treatment choice and monitoring response in patients. ctDNA analysis is a promising strategy for analyzing TKI resistance in oncogene-driven NSCLCs (12). Serial ctDNA analysis was necessary for detecting mutations of resistance as well as tumor heterogeneity and clonal evolution (Figure 3). We found ALK F1174C as the potential mechanism of brigatinib resistance.
Our study has some limitations. Due to the patient’s poor clinical condition during late stage treatment (performance score 2–3), no invasive examinations for tissue biopsy were applied. So we can only monitor the genetic alterations based on liquid biopsy only, which may miss some key information on the resistance mechanism from tissues biopsy. It’s reported that the median OS time from diagnosis in stage IV ALK-rearranged NSCLC patients was 81 months (13). However, the OS of our patient was only 4 years, and the most prolonged PFS of each TKI was 10 months. It indicated underlying ALK TKI resistance, probably mediated by off-target mechanisms such as bypass signaling, lineage changes, or Epithelial-Mesenchymal Transition (7,14).
Conclusions
We reported a patient benefited from the sequential use of ALK TKIs (crizotinib, brigatinib, and lorlatinib). A female patient with stage IIIB NSCLC detected EML4-ALK (E13:A20) rearrangement after chemotherapy and sequential radiotherapy and had a PR to crizotinib. ALK E1129V, F1174C, F1174L, F1174V, I1171T, and G1269A mutations emerged at crizotinib progression then responded to brigatinib therapy. Lorlatinib was used based on ctDNA analysis by NGS. Our case contributed to understanding the complexity of acquired resistance mechanisms in sequential ALK inhibitor therapy patients achieved durable clinical benefit.
Acknowledgments
The authors would like to thank Dr. Jiaqi Chu, Dr. Jie Yang, and Dr. Chunxiao Pan from Burning Rock Biotech for their suggestions in data analysis and manuscript writing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2838/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2838/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol 2015;16:e342-51. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Pan Y, Deng C, Qiu Z, et al. The Resistance Mechanisms and Treatment Strategies for ALK-Rearranged Non-Small Cell Lung Cancer. Front Oncol 2021;11:713530. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Dagogo-Jack I, Rooney M, Lin JJ, et al. Treatment with Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin Cancer Res 2019;25:6662-70. [Crossref] [PubMed]
- Tu J, Song LT, Liu RR, et al. Molecular inhibitory mechanism study on the potent inhibitor brigatinib against four crizotinib-resistant ALK mutations. J Cell Biochem 2019;120:562-74. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Aggarwal C, Thompson JC, Black TA, et al. Clinical Implications of Plasma-Based Genotyping With the Delivery of Personalized Therapy in Metastatic Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:173-80. [Crossref] [PubMed]
- Pacheco JM, Gao D, Smith D, et al. Natural History and Factors Associated with Overall Survival in Stage IV ALK-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:691-700. [Crossref] [PubMed]
- Urbanska EM, Sørensen JB, Melchior LC, et al. Changing ALK-TKI-Resistance Mechanisms in Rebiopsies of ALK-Rearranged NSCLC: ALK- and BRAF-Mutations Followed by Epithelial-Mesenchymal Transition. Int J Mol Sci 2020;21. [Crossref] [PubMed]



