
MiRNA-494 specifically inhibits SIRT3-mediated microglia activation in sepsis-associated encephalopathy
Introduction
Sepsis is a life-threatening multiple organ dysfunction disease caused by uncontrolled response of the body to infection (1), of which sepsis-associated encephalopathy (SAE) accounts for 70% of the total number of patients with sepsis. SAE is a diffuse brain dysfunction caused by systemic inflammation and is an important cause of increased mortality in patients in intensive care units (2,3). Patients who recover from SAE demonstrate various degrees of cognitive dysfunction persisting for months to years after discharge, which requires long-term medical intervention to restore function (4).
The pathogenesis of SAE has not been fully clarified, but current studies have confirmed that it is associated with abnormal regulation of the neuroimmune system (5-7). Microglia are the most common central nervous system resident immune cells that can regulate a variety of brain functions through cytokines and their interactions with neuronal and non-neuronal cells (8). However, cytokine dysregulation can cause neurotoxicity (9). Szöllősi et al. confirmed the activation of microglia in the brain in animal models of sepsis (10). In addition, aggravated neuroinflammation was shown to be caused by the activation of microglia (11). Moreover, numerous mediators were produced such as nitric oxide (NO), tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), oxygen free radicals, and excitatory neurotransmitters, which further amplified the inflammatory response and oxidative stress in the central nervous system, so that the neuronal injury was exacerbated (12,13). Therefore, regulation of microglial activation along with the inflammatory response and oxidative stress produced after their activation is essential for controlling SAE. Activation of recombinant sirtuin 3 (SIRT3), a key regulator located on mitochondria identified as being associated with multiple inflammations, significantly reduces microglial susceptibility to inflammatory stress (14,15). The expression of SIRT3 in turn has a correlation with the expression of a variety of microRNAs(miRNAs), such as miRNA-494-3p (16), miRNA-384 (17), and miRNA-195 (18). It has been shown that miRNAs are excellent biomarkers for diagnosis and prognosis, especially in encephalopathy, because they are small and penetrate the blood-brain barrier more easily than other biomolecules. However, few studies have screened SAE-related miRNAs and elaborated their pathogenesis in SAE. The identification of novel miRNAs as biomarkers may help to improve the diagnosis and prognosis of SAE and help to design new therapeutic approaches for this clinical presentation that generates diffuse brain dysfunction (19).
Therefore, it was hypothesized that miRNAs could regulate septic encephalopathy by regulating SIRT3 in microglia, then in turn, regulate microglial activation and oxidative stress responses. A rat SAE model was established in this study. The expression of inflammatory factors and SIRT3 in the brain tissue of SAE rats and the expression of mitochondrial function-related miRNAs were detected [miRNA-421 (20), miRNA-195, miRNA-708-5p (21), miRNA-384, miRNA-31 (22), and miRNA-494-3p], along with their possible regulatory relationship with inflammatory factors. Through in vitro cell experiments, it was confirmed that miRNA-494-3p regulates microglial activation and oxidative stress through SIRT3, which regulates the expression of inflammatory factors such as TNF-α and IL-6, and ultimately affects the progression of SAE. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1732/rc).
Methods
Experimental animals
Twenty male Wistar rats were purchased from the Animal Center of Xinjiang Medical University. Animal experiments were performed under a project license (No. 2018101) granted by Ethics Review Committee of the First People’s Hospital of Kashgar, in compliance with the institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Study methods
Establishment of the rat SAE model
Twenty male Wistar rats (200.0±12.3 g) were housed adaptively for 48 h in a 24 h light-dark cycle at a constant temperature of 23±1 ℃ and were provided with adequate food and water. The rats were randomly divided into 2 groups. The SAE model group (n=10) received an intraperitoneal injection of 5 mg/kg lipopolysaccharide (LPS) (Sosarbio, Beijing, China), while the control group (n=10) received an intraperitoneal injection of normal saline.
Monitoring of vital signs in rats
The basic vital signs of the rats were monitored at different time points after model establishment. For the measurement of body temperature, the rats were fixed and an electronic thermometer was inserted about 2 cm into the anus of the rats until the value of the electronic thermometer was stable at 0 h, 1 h, 3 h, 6 h, and 8 h after model establishment. A prompt sound was given, and the body temperature data displayed on the screen were read. For the measurement of food intake, quantitative food was weighed to feed the rats before model establishment, and the remaining food was weighed after the rats were sacrificed at 8 h. The difference was the food intake. In terms of signs, physiological manifestations such as drowsiness and piloerection were monitored.
Rat microglial cell culture and transfection
Rat microglia (Fame Biotechnology Co., Ltd., Shanghai, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) complete medium (Gibco, Carlsbad, California, USA). A miRNA-494 lentiviral expression vector (Jikai Gene, Shanghai, China) was constructed and transfected into microglia with PLVX-mCMV-ZsGreen-PGK-Puro-homo-miRNA-494 virus multiplicity of infection (MOI) = 20, and a negative control group (NC group) was set up. After the addition of lentivirus, the cells were incubated in a 37 ℃, 5% CO2 incubator (Likang Bio-Medical Technology Holding Co., Ltd., Hong Kong, China) for 16 h. Fresh DMEM complete medium (Gibco) was replaced for 72 h before harvesting the cells.
Immunohistochemical detection of Ionized calcium bindingadaptor molecule-1 (Iba-1) and SIRT3
Rat brain tissues were obtained 8 h after modeling, and a portion was cut and placed in a specimen bottle containing 4% paraformaldehyde and stored and fixed in a refrigerator at 4 ℃ for 12 to 24 h. The tissue was placed in a dehydration and embedding cassette, rinsed and dehydrated in gradient ethanol at room temperature, and cleared in xylene. The tissues were then transferred to paraffin I for wax immersion and embedded with paraffin II. After sectioning, slices were baked at 56 ℃, deparaffinized in xylene, dehydrated in graded ethanol, and rinsed with phosphate-buffered saline (PBS, pH 7.4) for 3×3 min. The slides were immersed in boiling sodium citrate buffer solution, heated in a pressure cooker to air jet for 2 min, taken out after the pressure cooker naturally cooled to room temperature, and then rinsed with PBS for 3×3 min. A 3.0% H2O2 solution was applied to each section, incubated at room temperature for 10 min, and rinsed with PBS for 3×3 min. A total of 50 µL each of rabbit anti-AIF1/Iba-1 antibody (Bioss, Beijing, China) and rabbit anti-SIRT3 antibody (Bioss, Beijing, China) diluted at a concentration of 1:500 was added to the sections and incubated for 2–3 h at room temperature, followed by washing with PBS 3×3 min. A total of 50 µL of goat anti-rabbit IgG HampL/HRP antibody (Bioss, Beijing, China) was added dropwise to each section, incubated at room temperature for 30 min, and rinsed with PBS for 3×3 min. A total of 100 µL of DAB chromogenic solution (3,3'-diainobenzidine hydrochloride) was added to each section, washed, and counterstained with hematoxylin, differentiated with 0.1% hydrochloric acid alcohol, and washed with PBS to return to blue. Sections were dehydrated in gradient alcohol then dried, cleared in xylene, and mounted in neutral gum.
Enzyme-linked immuno sorbent assay (ELISA) for IL-6 and TNF-α
At 8 h after model establishment, rat brain tissues were obtained, a part of which was cut for tissue homogenization, and 4 ℃ PBS was added to prepare 10% tissue homogenate, which was centrifuged with a high-speed refrigerated centrifuge (Zhongke Zhongjia Scientific Instrument Co., Ltd., Anhui, China) for 10 min. The supernatant was obtained, dispensed, and cryopreserved. For cells after transfection, the supernatant was collected and cryopreserved. The kit (Sosarbio, Beijing, China) was placed at room temperature for 40 min and shaken well. For sample addition after dilution, the control wells and sample wells to be tested were set, then samples were added without touching the well walls. After shaking gently, the plate was covered with sealing membrane, incubated at 37 ℃ for 1 h, then washed. Subsequently, microplate labeling reagent (Sosarbio, Beijing, China) (control wells) was added, then the plate was covered by sealing membrane and incubated at 37 ℃ for 0.5–1 h followed by washing. Substrate solution was added and mixed well, which was then reacted at 37 ℃ in the dark for 15 min for color development. The terminator was added to terminate the reaction, and the optical density (OD) value at 450 nm on a microplate reader was determined (Thermo Scientific, Waltham, Massachusetts, USA).
Relative expression of miRNA-4215p, miRNA-1953p, miRNA708-5p, miRNA384, miRNA-31a-5p, miRNA-494-3p, and SIRT3 mRNA by RT-qPCR
Total RNA was extracted by the TRIzol method. RNA concentration was determined by a UV spectrophotometer (Puxie General Instruments Co., Ltd., Beijing, China) and stored at −80 ℃ until use. First strand cDNA was obtained by reverse transcription reagent guidance. The qPCR assays for miRNA levels were performed guided by the corresponding primers (Table 1) by using U6 as an internal reference. Reaction program: 95 ℃, 3 min; 95 ℃, 5 s; 56 ℃, 10 s; 72 ℃, 25 s; 39 cycles; 65 ℃, 5 s; 95 ℃, 50 s. Under the guidance of the corresponding primers, β-actin was set as an internal reference for the detection of SIRT3 and NMNAT1. Reaction program: 95 ℃, 5 min, 95 ℃, 15 s; 56 ℃, 15 s, 72 ℃, 60 s, 40 cycles. The relative expression was calculated by the 2−∆∆Ct method.
Table 1
| Primer | 5'-3' |
|---|---|
| Rat-SIRT3-F | TCAGCAGTATGACATCCCGTACCC |
| Rat-SIRT3-R | CGTGAAGCAGCCGAAGGAAGTAG |
| Rat-beta-actin-F | TGTGATGGTGGGTATGGGT |
| Rat-beta-actin-R | TGTAGAAAGTGTGGTGCCAA |
| Rat-miR708-5p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCCCAGC |
| Rat-miR708-5p-F | GCGCGAAGGAGCTTACAATCTA |
| Rat-miR494-3p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGAGGT |
| Rat-miR494-3p-F | GCGTGAAACATACACGGGA |
| Rat-Mic195-3p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGGAGC |
| Rat-Mic195-3p-F | CGCCAATATTGGCTGTGCT |
| Rat-Mic31a-5p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAGCTATG |
| Rat-Mic31a-5p-F | ACTCCAGCTGGGAGGCAAGATGCTGG |
| Rat-Mic421-5p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAACAA |
| Rat-Mic421-5p-F | CGCGGGCCTCATTAAATGT |
| Rat-U6-F | TGAAGACAGCCCAGAATCA |
| Rat-U6-R | GGCATCCTCCAGAACTTGT |
| Rat-Mic384-5p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGTGAACA |
| Rat-Mic384-5p-F | ACTCCAGCTGGGATTCCTAGAAAT |
| Rat-miR-reverse | AGTGCAGGGTCCGAGGTATT |
Western blot
Total protein was extracted and quantified by Bicinchoninic acid protein quantification, and 40 ug of total protein from each group was separated by sodium dodecyl sulphate (SDS)-polyacrylamide gels (4% stacking gel and 10% separating gel) and transferred to Polyvinylidene fluoride (PVDF) membranes. Red staining determined whether the membrane transfer was successful. Membranes were blocked with 5% non-fat dry milk at room temperature. The next day, the blocking solution was discarded and the membranes were washed. Rabbit anti-NMNAT1 antibody (Bioss, Beijing, China) and rabbit anti-SIRT3 antibody (Bioss) were diluted with 1× PBS solution according to the ratio of antibody instructions, added to the membranes, and incubated at 4 ℃ for 12 h. After washing the membranes with Tris Buffered Saline with Tween 3 times, the membranes were incubated with HRP-labeled goat anti-rabbit IgG HampL/HRP antibody (Bioss) (1:5,000) at room temperature for 2 h. After washing the membranes again 3 times, enhanced chemiluminescence (ECL) chemiluminescence substrate (Sosarbio, Beijing, China) was added and exposed for development. A protein band scanner (Liuyi Biotechnology Co., Ltd., Beijing, China) was used for scanning and densitometric analysis.
Statistical analysis
Data analysis was performed by SPSS 16.0 statistical software. The experimental results were expressed as mean ± standard deviation (mean ± SD). The comparison of multiple groups of data was analyzed by one-way ANOVA. The homogeneity test of variance was also performed. P<0.05 indicated statistical significance.
Results
Monitoring of vital signs in rats
A rat model of septic encephalopathy was established by intraperitoneal injection of LPS, and the body temperature, food intake, and signs of the rats were monitored at different time points. The results showed that there was no significant difference in body temperature between the SAE model group (37.01±0.22 ℃) and the control group (36.78±0.30 ℃) (P>0.05). However, the food intake of the rats in the model group (2.94±0.63 g) was significantly lower than that of the control group (12.86±1.30 g) (P<0.05). The rats in the model group showed physiological phenomena of drowsiness and piloerection 3 h after modeling (Figure 1).
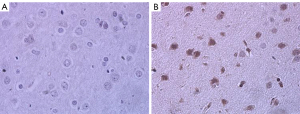
Activation of microglia and expression of inflammatory factors in brain tissue
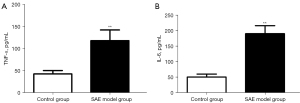
The expression and distribution of microglial activation marker Iba-1 in brain tissue were detected by immunohistochemistry. The results showed that there was positive expression of Iba-1 in the brain tissue of rats in the SAE model group, and there was more expression than that in the control group (Figure 2). ELISA was used to detect the expression of inflammatory factors in the brain tissue of septic encephalopathy rats, and the results showed that TNF-α expression in the SAE model group (118.90±9.10 pg/mL) was significantly higher than that in the control group (43.52±2.68 pg/mL) (P<0.01). IL-6 expression in the SAE model group (190.90±9.68 pg/mL) was significantly higher than that in the control group (51.64±3.34 pg/mL) (P<0.01) (Figure 3). It was confirmed that microglia were activated and inflammatory factors IL-6 and TNF-α were released in the brain tissue of SAE rats.
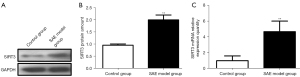
Expression of SIRT3 and miRNAs in brain tissue
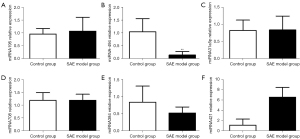
The relative expression of SIRT3 mRNA and protein in rat brain tissue was detected, and the results showed that SIRT3 protein expression and relative mRNA expression in the SAE model group were significantly higher than those in the control group (P<0.01) (Figure 4). The expression of miRNAs in brain tissue was detected, and the results showed that the relative expression of miRNA-494 in the SAE model group was significantly lower than that in the control group (P<0.01). The relative expression of miRNA-421 in the SAE model group was significantly higher than that in the control group (P<0.01). There was no significant difference in the relative expression of miRNA-195, miRNA-31a5p, miRNA-708, and miRNA384 between the 2 groups (P>0.05) (Figure 5).
SIRT3 expression in microglia and microglial activation after miRNA-494 transfection
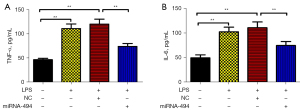
After miRNA-494 was transfected into microglia, the cells were treated with LPS, and the supernatant was collected to detect the expression of TNF-α and IL-6. The results showed that the expression levels of TNF-α and IL-6 in the supernatant of cells transfected with miRNA-494 were significantly reduced (P<0.01) (Figure 6). The expression of Iba-1 and SIRT3 in the cells was detected, and the results showed that the expression levels of SIRT3 and Iba-1 protein in microglia after miRNA-494 transfection were significantly lower than those in the NC group (P<0.01) (Figure 7).

Discussion
SAE is one of the major causes of increased mortality and poor prognosis in sepsis patients. Patients have been found to have dysregulation of neuroendocrine immune factors, and this dysregulated neuroinflammation and ischemic injury are the main causes of brain dysfunction. Dysfunction of these neuromodulatory mechanisms could impair systemic immune responses, including those of neutrophils, macrophages/monocytes, dendritic cells, and T lymphocytes, which ultimately induce a vicious cycle between brain injury and gradually abnormal immune responses (23). It is well known that inflammation is able to increase cytokine transcription of β-Interleukin I (IL-1β), TNF-α, and IL-6, while IL-1β can activate microglia and is neurotoxic during the production and release of NO and reactive oxygen species (ROS) (24-26). TNF-α can lead to neutrophil infiltration, neuronal apoptosis, and brain edema in brain tissue (26). In addition, it is capable of causing neurotoxicity in the early stages of neuroinflammation (27). Meanwhile, IL-6 can induce cyclooxygenase-2 (COX-2) expression, which in turn increases vasodilatory prostaglandin I2 (PGI 2) (26,28), leading to potential fever and behavioral disturbances (24). SAE is characterized by activation of the inflammatory cascade and increased cytokine levels. SAE is accompanied by excessive activation of microglia and the release of a large number of inflammatory factors and NO, and the massive release of these factors will cause the body to produce excessive inflammatory responses and oxidative stress, thus further exacerbating the progression of SAE (29,30). Iba-1 is a marker of microglial activation (31). Our study demonstrated that the expression of Iba-1 was significantly increased in the brain tissue of SAE model rats, along with the release of large amounts of the inflammatory cytokines TNF-α and IL-6. Meanwhile, oxidative stress and inflammatory responses in the brain tissue of SAE rats could play an important role in the pathogenesis of mitochondrial damage in brain tissue at the early stages of sepsis, which could lead to mitochondrial dysfunction and metabolic abnormalities (32). SIRT3 could play an important role in maintaining mitochondrial function by exerting deacetylation functions in mitochondria. Jiang et al. showed that overexpression of SIRT3 decreased ROS levels in neural stem cells (NSCs) and alleviated cell damage caused by oxidative stress (33). Zeng et al. also demonstrated that SIRT3 overexpression suppresses LPS-induced pericyte loss and inflammatory infiltration of neutrophils/macrophages in cardiopulmonary tissues to attenuate inflammatory injury (34). It was confirmed in our study that SIRT3 expression was up-regulated in the brain tissue of SAE model rats, suggesting that SIRT3 expression may be up-regulated under inflammatory and oxidative stress conditions so as to maintain mitochondrial function and further counteract the oxidative stress caused by mediating inflammation. Therefore, regulation of SIRT3 may be a key factor in the regulation of microglial activation and oxidative stress.
MiRNAs are small non-coding RNAs which regulate post-transcriptional gene expression levels by inducing mRNA translational repression or degradation (35). MiRNAs have been extensively studied in many diseases and their regulatory roles have been confirmed (36). It is important that miRNAs are highly conserved in mammals and are key players in cellular processes such as cell differentiation, growth, proliferation, apoptosis, metabolism, and cell homeostasis (35,37,38). In the brain, miRNAs, can play a role in regulating the expression levels of target genes through the blood-brain barrier (39,40). Some studies have suggested that miRNA421, miRNA195, and miRNA708 may be involved in the regulation of SIRT3 expression and affect its acetylation level (17,20,21,32,41). However, there are no reports that SIRT3 is associated with microglial activation. Our results confirmed that miRNA-494 expression was significantly decreased in the brain tissue of SAE model rats. Additionally, overexpression of miRNA-494 significantly down-regulated SIRT3 expression in microglia, while significantly reducing the expression of inflammatory cytokines TNF-α and IL-6 and the microglial activation marker Iba-1 in LPS-induced microglial inflammation. It is speculated that miRNA-494 may be involved in regulating LPS-induced microglial activation and inflammatory cytokine production through SIRT3.
SAE is a common and serious complication of the dysregulated host response in sepsis. The molecular mechanism of its pathophysiology is not fully understood, but changes in brain metabolism, hyperinflammatory phenotype, and immune responses could play a central role in the development and progression of SAE. Microglia are important players in the function of brain immune defenses and in inflammation (19). It was confirmed in this study that the biomarker miRNA-494 may help clinicians diagnose and predict SAE and explain its regulatory mechanism. However, a single biomarker may not be sufficient to diagnose this heterogeneous syndrome, while the combination of biomarkers may improve the performance of diagnostic methods and reduce the future morbidity of SAE patients. The basic research data in this study may contribute to the further development of new treatments for SAE and the discovery of diagnostic markers.
Acknowledgments
Funding: The study was supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (2016D01C023).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1732/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1732/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1732/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. 2018101) granted by Ethics Review Committee of the First People’s Hospital of Kashgar, in compliance with the institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Schuler A, Wulf DA, Lu Y, et al. The Impact of Acute Organ Dysfunction on Long-Term Survival in Sepsis. Crit Care Med 2018;46:843-9. [Crossref] [PubMed]
- Helbing DL, Böhm L, Witte OW. Sepsis-associated encephalopathy. CMAJ 2018;190:E1083. [Crossref] [PubMed]
- Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol 2014;13:630-6. [Crossref] [PubMed]
- Wilson JX, Young GB. Progress in clinical neurosciences: sepsis-associated encephalopathy: evolving concepts. Can J Neurol Sci 2003;30:98-105. [Crossref] [PubMed]
- Heming N, Mazeraud A, Verdonk F, et al. Neuroanatomy of sepsis-associated encephalopathy. Crit Care 2017;21:65. [Crossref] [PubMed]
- Mazeraud A, Pascal Q, Verdonk F, et al. Neuroanatomy and Physiology of Brain Dysfunction in Sepsis. Clin Chest Med 2016;37:333-45. [Crossref] [PubMed]
- Greenhalgh AD, David S, Bennett FC. Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci 2020;21:139-52. [Crossref] [PubMed]
- van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 2010;375:773-5. [Crossref] [PubMed]
- Szöllősi D, Hegedűs N, Veres DS, et al. Evaluation of Brain Nuclear Medicine Imaging Tracers in a Murine Model of Sepsis-Associated Encephalopathy. Mol Imaging Biol 2018;20:952-62. [Crossref] [PubMed]
- Sharshar T, Polito A, Checinski A, et al. Septic-associated encephalopathy - everything starts at a microlevel. Crit Care 2010;14:199. [Crossref] [PubMed]
- Michels M, Danielski LG, Dal-Pizzol F, et al. Neuroinflammation: microglial activation during sepsis. Curr Neurovasc Res 2014;11:262-70. [Crossref] [PubMed]
- Lv M, Liu Y, Zhang J, et al. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience 2011;176:162-72. [Crossref] [PubMed]
- Lee HJ, Kang MG, Cha HY, et al. Effects of Piceatannol and Resveratrol on Sirtuins and Hepatic Inflammation in High-Fat Diet-Fed Mice. J Med Food 2019;22:833-40. [Crossref] [PubMed]
- Huang D, Liu M, Jiang Y. Mitochonic acid‐5 attenuates TNF‐α‐mediated neuronal inflammation via activating Parkin‐related mitophagy and augmenting the AMPK–Sirt3 pathways. J Cell Physiol 2019;234:22172-82. [Crossref] [PubMed]
- Geng L, Zhang T, Liu W, et al. miR-494-3p modulates the progression of in vitro and in vivo Parkinson’s disease models by targeting SIRT3. Neurosci Lett 2018;675:23-30. [Crossref] [PubMed]
- Zhang B, Cui S, Bai X, et al. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age (Dordr) 2013;35:2237-53. [Crossref] [PubMed]
- Fan X, Xiao M, Zhang Q, et al. miR-195-Sirt3 Axis Regulates Angiotensin II-Induced Hippocampal Apoptosis and Learning Impairment in Mice. Psychol Res Behav Manag 2019;12:1099-108. [Crossref] [PubMed]
- Osca-Verdegal R, Beltrán-García J, Pallardó FV, et al. Role of microRNAs As Biomarkers in Sepsis-Associated Encephalopathy. Mol Neurobiol 2021;58:4682-93. [Crossref] [PubMed]
- Yang P, Zhang M, Liu X, et al. MicroRNA-421 promotes the proliferation and metastasis of gastric cancer cells by targeting claudin-11. Exp Ther Med 2017;14:2625-32. [Crossref] [PubMed]
- Huang S, Guo H, Cao Y, et al. MiR-708-5p inhibits the progression of pancreatic ductal adenocarcinoma by targeting Sirt3. Pathol Res Pract 2019;215:794-800. [Crossref] [PubMed]
- Kao YY, Chou CH, Yeh LY, et al. MicroRNA miR-31 targets SIRT3 to disrupt mitochondrial activity and increase oxidative stress in oral carcinoma. Cancer Lett 2019;456:40-8. [Crossref] [PubMed]
- Ren C, Yao RQ, Zhang H, et al. Sepsis-associated encephalopathy: a vicious cycle of immunosuppression. J Neuroinflammation 2020;17:14. [Crossref] [PubMed]
- Chaudhry N, Duggal AK. Sepsis Associated Encephalopathy. Adv Med 2014;2014:762320. [Crossref] [PubMed]
- Sonneville R, Verdonk F, Rauturier C, et al. Understanding brain dysfunction in sepsis. Ann Intensive Care 2013;3:15. [Crossref] [PubMed]
- Pampín-Huerta FR, Lozano-Requelme ML, Galeiras-Vázquez RM, et al. Encefalopatía asociada a la sepsis como presentación de una infección urinaria bacteriémica por Proteus mirabilis. Infectio 2016;20:169-71. [Crossref]
- O'Callaghan JP, Sriram K, Miller DB. Defining "neuroinflammation". Ann N Y Acad Sci 2008;1139:318-30. [Crossref] [PubMed]
- Santos-Junior NN, Catal O C, Costa L, et al. Experimental sepsis induces sustained inflammation and acetylcholinesterase activity impairment in the hypothalamus. J Neuroimmunol 2018;324:143-8. [Crossref] [PubMed]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 2007;8:57-69. [Crossref] [PubMed]
- Olmos G, Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014;2014:861231. [Crossref] [PubMed]
- Waller R, Baxter L, Fillingham DJ. Iba-1-/CD68+ microglia are a prominent feature of age-associated deep subcortical white matter lesions. PLoS One 2019;14:e0210888. [PubMed]
- Zhang X, Ji R, Liao X, et al. MicroRNA-195 Regulates Metabolism in Failing Myocardium Via Alterations in Sirtuin 3 Expression and Mitochondrial Protein Acetylation. Circulation 2018;137:2052-67. [Crossref] [PubMed]
- Jiang DQ, Wang Y, Li MX, et al. SIRT3 in Neural Stem Cells Attenuates Microglia Activation-Induced Oxidative Stress Injury Through Mitochondrial Pathway. Front Cell Neurosci 2017;11:7. [Crossref] [PubMed]
- Zeng H, He X, Tuo QH, et al. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2α/Notch3 pathways. Sci Rep 2016;6:20931. [Crossref] [PubMed]
- Almeida MI, Reis RM, Calin GA. MicroRNA history: discovery, recent applications, and next frontiers. Mutat Res 2011;717:1-8. [Crossref] [PubMed]
- Geaghan M, Cairns MJ. MicroRNA and Posttranscriptional Dysregulation in Psychiatry. Biol Psychiatry 2015;78:231-9. [Crossref] [PubMed]
- Huang W. MicroRNAs: Biomarkers, Diagnostics, and Therapeutics. Methods Mol Biol 2017;1617:57-67. [Crossref] [PubMed]
- Wang J, Chen J, Sen S. MicroRNA as Biomarkers and Diagnostics. J Cell Physiol 2016;231:25-30. [Crossref] [PubMed]
- Szilágyi B, Fejes Z, Pócsi M, et al. Role of sepsis modulated circulating microRNAs. EJIFCC 2019;30:128-45. [PubMed]
- Puskarich MA, Nandi U, Shapiro NI, et al. Detection of microRNAs in patients with sepsis. Journal of Acute Disease 2015;4:101-6. [Crossref]
- Zhang J, Zhu Y, Hu L, et al. miR-494 induces EndMT and promotes the development of HCC (Hepatocellular Carcinoma) by targeting SIRT3/TGF-β/SMAD signaling pathway. Sci Rep 2019;9:7213. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


