
Comedication with corticosteroids and nonsteroidal antiphlogistics does not affect PD-L1 expression in non-small cell lung cancer
Introduction
Programmed death-ligand 1 (PD-L1) expression, despite its limitations, plays a crucial role in the choosing of immunotherapy in first-line treatment of metastatic non-small cell lung cancer (NSCLC), as well as in the consolidation with durvalumab therapy after chemoradiotherapy in stage III NSCLC (1). Further work has shown, however, that predicting the effectiveness of immunotherapy in NSCLC may be affected by other parameters (2). These predictive markers may include also comedication with corticosteroids or non-steroidal anti-inflammatory drugs (NSAIDs), as evidenced by some previous publications on this topic (3,4). A question therefore arises as to whether the level of PD-L1 expression might be influenced in some way by use of such a comedication. According to the available literature, certain common genetic and molecular pathways can be traced to support this idea. Regarding corticosteroids, for example, the influence of PD-L1 expression on dendritic cells (DCs) has been previously described through the glucocorticoid-induced leucine zipper (GILZ) system, which is activated by the use of corticosteroids (5,6). Recently, a comprehensive review has also been published pointing to a number of links between PD-L1 and corticosteroids (7). Other articles describe a relationship of the RAC(Rho family)-alpha serine/threonine-protein kinase (AKT)/signal transducer and activator of transcription 3 (STAT3) or nuclear factor kappa B (NF-κB) pathway to cyclooxygenase-2 (COX2) (8,9). These pathways are also related to the level of PD-L1 expression (10-12). Moreover, recent article suggested decrease expression of PD-L1 in colon tumors in mouse due to of NSAID administration (13).
The aim of this work was therefore to determine whether the level of PD-L1 expression may be related to the using of corticosteroids or NSAIDs as of the date of performing a lung cancer biopsy. Based on the above data, we hypothesize that corticosteroids and NSAIDs used could decrease PD-L1 expression. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-260/rc).
Methods
Patients
Clinical data of patients with histologically confirmed locally advanced or advanced (stage III or IV) NSCLC that were assigned to the LUng CAncer focuS (LUCAS) registry were retrospectively analyzed. A national register, LUCAS is a non-interventional post-registration database of epidemiological and clinical data of patients with lung cancer in the Czech Republic (from high volume centers that allow participation in this project).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Palacky University in Olomouc on June 11, 2018 (under reference number 63/18 MEK 13) and individual consent for this retrospective analysis was waived.
The patients’ data were obtained from seven pneumo-oncology departments in the Czech Republic between 2018 and 2021—all patients with NSCLC stage III or IV with known PD-L1 expression were inclusion in the study. Information regarding comedication with corticosteroids or NSAIDs as of the date of biopsy was found in medical databases and also entered into the LUCAS register. PD-L1 expression levels and baseline patient characteristics were obtained from the LUCAS registry. Only patients with a corticosteroid dose equivalent to 10 mg prednisolone and above were counted. Acetylsalicylic acid was also included among the NSAIDs, but topical drugs were not included among the NSAIDs.
PD-L1 expression estimation
Immunohistochemical staining was performed on automated immunostainers (different types in different laboratories) on formalin-fixed (10% neutral buffered formalin; fixation time 24 h), paraffin-embedded tissue sections (3 nm thick). All laboratories used PD-L1 clone 22C3 pharmDx (Dako, Carpinteria, California, USA). The specimens were visualized using the ultraView Universal DAB Detection Kit (Ventana Medical Systems, Oro Valley, Arizona, USA).
Statistical analysis
Continuous parameters were described by valid N, mean with standard deviation (SD), and median with 5th and 95th percentile. Categorical data were described by absolute and relative frequencies. PD-L1 expression was analyzed categorically (<1%; 1–49%; ≥50%) at cut-off points that are clinically valuable for immunotherapy. Independence of two categorical parameters was tested by Pearson’s chi-square test. Statistically significant results (relationships) were those for which P<0.05. The analysis was performed in IBM SPSS Statistics 24.
Results
Patient characteristics
A total of 1,148 patients were evaluated (20 patients were excluded due to unknown PD-L1 expression, in 1 included patient data about comedications were unknown) (Figure 1). The analyzed dataset consisted 61% of men. Mean age was 67.7 years (median 68.8 years). Among the patients, 31% were younger than 65 years of age at the time of diagnosis. Eighty-nine patients (7.8%) had tumors of types other than adenocarcinoma or squamous: 59 were NSCLC not otherwise specified (NOS), 22 were adenosquamous, and 8 were NSCLC neuroendocrine differentiation. Baseline patient characteristics are described in Table 1.
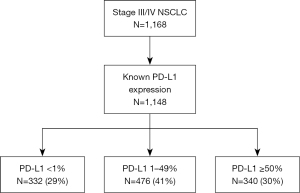
Table 1
| Parameter | Category | N (%) |
|---|---|---|
| Sex | Male | 703 (61.2) |
| Female | 445 (38.8) | |
| Age at diagnosis | <65 years | 360 (31.4) |
| ≥65 years | 788 (68.6) | |
| Smoking status | Non-smoker | 162 (14.1) |
| Ex-smoker | 374 (32.6) | |
| Smoker | 576 (50.2) | |
| Unknown | 36 (3.1) | |
| Performance status | 0 | 245 (21.3) |
| 1 | 656 (57.1) | |
| ≥2 | 205 (17.9) | |
| Unknown | 42 (3.7) | |
| Tumor type | Adenocarcinoma | 654 (57.0) |
| Squamous carcinoma | 405 (35.3) | |
| Other | 89 (7.8) | |
| Stage (TNM 8th edition) | III | 399 (34.8) |
| IV | 749 (65.2) |
Performance status according to Eastern Cooperative Oncology Group classification. TNM, tumour, node and metastasis.
Mean PD-L1 expression was 28.7% and median was 10.0%. Among all evaluated patients, 29% had PD-L1 expression <1% (PD-L1 negative), 41% had PD-L1 expression between 1% and 49%, and 30% of patients had PD-L1 expression ≥50%.
The basic characteristics of comedication values are described in Table 2.
Table 2
| Comedication | Using | N (%) |
|---|---|---|
| Corticosteroids† | No | 1,031 (89.9) |
| Yes | 116 (10.1) | |
| NSAIDs† | No | 838 (73.1) |
| Yes | 309 (26.9) |
†, for 1 patient, there was no information about comedication. NSAIDs, non-steroidal anti-inflammatory drugs.
Relationship between basic patient characteristics and PD-L1 expression
PD-L1 expression was tested as a categorical parameter. There were no statistically significant relationships between basic patient characteristics and PD-L1 expression. Results are summarized in Table 3.
Table 3
| Parameter | Category | PD-L1 <1%, n (%) | PD-L1: 1–49%, n (%) | PD-L1 ≥50%, n (%) | P value |
|---|---|---|---|---|---|
| Sex | Men | 204 (29.0) | 294 (41.8) | 205 (29.2) | 0.910 |
| Women | 128 (28.8) | 182 (40.9) | 135 (30.3) | ||
| Age at diagnosis | <65 years | 99 (27.5) | 155 (43.1) | 106 (29.4) | 0.708 |
| ≥65 years | 233 (29.6) | 321 (40.7) | 234 (29.7) | ||
| Smoking status | Non-smoker | 53 (32.7) | 67 (41.4) | 42 (25.9) | 0.679 |
| Ex-smoker | 112 (29.9) | 152 (40.6) | 110 (29.4) | ||
| Smoker | 159 (27.6) | 240 (41.7) | 177 (30.7) | ||
| Performance status | 0 | 66 (26.9) | 102 (41.6) | 77 (31.4) | 0.120 |
| 1 | 199 (30.3) | 256 (39.0) | 201 (30.6) | ||
| ≥2 | 56 (27.3) | 100 (48.8) | 49 (23.9) | ||
| Tumor type | Adenocarcinoma | 193 (29.5) | 252 (38.5) | 209 (32.0) | 0.113 |
| Squamous | 118 (29.1) | 183 (45.2) | 104 (25.7) | ||
| Other | 21 (23.6) | 41 (46.1) | 27 (30.3) | ||
| Stage | III | 116 (29.1) | 164 (41.1) | 119 (29.8) | 0.984 |
| IV | 216 (28.8) | 312 (41.7) | 221 (29.5) |
Performance status according to Eastern Cooperative Oncology Group classification. PD-L1, programmed death-ligand 1.
Relationship between comedications and PD-L1 expression
There were no statistically significant relationships between the use of corticosteroids or NSAIDs at the time of lung cancer biopsy and PD-L1 expression. Results are summarized in Table 4.
Table 4
| Comedication | Using | PD-L1 <1%, n (%) | PD-L1: 1–49%, n (%) | PD-L1 ≥50%, n (%) | P value |
|---|---|---|---|---|---|
| Corticosteroids | No | 297 (28.8) | 426 (41.3) | 308 (29.9) | 0.780 |
| Yes | 35 (30.2) | 50 (43.1) | 31 (26.7) | ||
| NSAIDs | No | 239 (28.5) | 349 (41.6) | 250 (29.8) | 0.864 |
| Yes | 93 (30.1) | 127 (41.1) | 89 (28.8) |
NSAIDs, non-steroidal anti-inflammatory drugs; PD-L1, programmed death-ligand 1.
Discussion
Although corticosteroids and perhaps also NSAIDs may be predictors for the effectiveness of immunotherapy, in our study we demonstrated no relationship between these comedications and the level of PD-L1 expression. We therefore rejected our hypothesis of reduced PD-L1 expression by these drugs. In our opinion, this result has a significant clinical impact, as our data show that at the time of biopsy, it is not necessary to take into account the use of corticosteroids/NSAIDs by patients due to possible concerns about affecting PD-L1 expression.
To the best of our knowledge, this is the first clinical study investigating the effect of comedication with a higher dose of corticosteroid (equivalent to 10 mg or more of prednisolone) on PD-L1 expression on tumor cells. We have found several works in the literature that deal with similar issues on cell lines. Despite there being some known common metabolic pathways that affect corticosteroids and at the same time contribute to the control of PD-L1 expression, the effect of corticosteroids probably depends upon several factors (5,14,15). First, it likely depends upon the type of cells examined for PD-L1 expression. For example, upon administration of dexamethasone on cell lines, an effect has been described of PD-L1 expression on natural killer (NK) cells versus no effect of PD-L1 expression on DCs (14-16). Furthermore, it probably depends upon the dose of corticosteroids administered. As shown from two studies investigating the effect of inhaled corticosteroids (ICS) on PD-L1 expression on respiratory epithelial cells, such effect was manifested only at their high administered dose (17,18). By contrast, another study demonstrated a beneficial effect of even low-dose corticosteroids in reducing PD-L1 expression on a tumor cell line (19). The authors of this work, however, worked with gastric cancer, hepatocellular cancer, and pancreatic cancer cell lines and not with NSCLC tumor cell lines. Thus, it is possible that the effect of corticosteroids on PD-L1 expression on tumor cells may be different also in various tumor types.
Concerning NSAIDs, we also can compare our data only with the results of studies on cell lines. Botti et al. demonstrated an effect of celecoxib administration in reducing PD-L1 expression on selected melanoma cell lines (20). Although Shimizu et al. also showed a correlation between COX-2 and PD-L1 expression in lung cancer resections, celecoxib administration did not affect PD-L1 expression in lung cancer cell lines (21). Tang et al. explained this by the need to simultaneously inhibit epidermal growth factor receptor (EGFR), as demonstrated in their study on lung cancer cell lines (22). In a study on breast cancer cell lines, Liang et al. pointed to the need for higher doses of nimesulide to suppress PD-L1 expression (23). It is possible, therefore, that any effect of NSAIDs on PD-L1 expression will differ according to the type of tumor and dose of NSAIDs used. This could explain differences apparent in comparing the results of our work with those from the study by Zhang et al., who on lung cancer cell lines described a decrease in PD-L1 expression when aspirin was administered (18). We did not confirm this in our study in patients with NSCLC administered NSAIDs, including aspirin. This might be explained by the possibly different effects of various NSAIDs in the patients in our study. However, an effect cannot be ruled out of aspirin dosages reported in the study by Zhang et al. significantly exceeding those commonly administered in clinical practice (100 mg/day) (18,24).
One of the limitations of our study is the retrospective data collection in the LUCAS registry, which also is limited to large clinical centers. This can lead to some bias when enrolling patients. On the other hand, we believe that, due to the robustness of our group, the risk of non-representativeness of the data within the Czech Republic is minimal. There was also no external validation of the measured laboratory parameters and PD-L1 expression, but all these values were determined by accredited methods within accredited workplaces ensuring routine internal and external quality control. The use of different antibodies to detect PD-L1 also should not play a role within our cohort, as all laboratories in our project used the same clone of PD-L1 antibodies. Last but not least, due to the retrospective nature of the study, the exact timing of NSAIDs and corticosteroids is unknown. Theoretically, if the administration time is too short in some patients, the effect of these comedications may not be apparent. The strength of our study is the large number of evaluated patients, which bolsters robustness of the statistical determinations.
Conclusions
According to our data, treatment with corticosteroids or NSAIDs during biopsy does not affect the expression of PD-L1 and it is therefore not necessary to take this treatment into account in this regard. We also did not demonstrate any effect of common demographic data, including smoking, on PD-L1 expression levels.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-260/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-260/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-260/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-260/coif). M Svaton received honoraria by Astra Zeneca, Roche, MSD, BMS, Takeda, Novartis (consulting fees, lectures, Advisory Boards) and also is supported by academic institutional grant. M Bratova had presentations and lectures for BMS, MSD and Astra Zeneca in last 36 months. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Palacky University in Olomouc on June 11, 2018 (under reference number 63/18 MEK 13) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer 2020;20:1185. [Crossref] [PubMed]
- Svaton M, Zemanova M, Zemanova P, et al. Impact of Concomitant Medication Administered at the Time of Initiation of Nivolumab Therapy on Outcome in Non-small Cell Lung Cancer. Anticancer Res 2020;40:2209-17. [PubMed]
- Arbour KC, Mezquita L, Long N, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2872-8. [Crossref] [PubMed]
- Cathelin D, Met Ö, Svane IM. Silencing of the glucocorticoid-induced leucine zipper improves the immunogenicity of clinical-grade dendritic cells. Cytotherapy 2013;15:740-9. [Crossref] [PubMed]
- Cohen N, Mouly E, Hamdi H, et al. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood 2006;107:2037-44. [Crossref] [PubMed]
- Adorisio S, Cannarile L, Delfino DV, et al. Glucocorticoid and PD-1 Cross-Talk: Does the Immune System Become Confused? Cells 2021;10:2333. [Crossref] [PubMed]
- De Cicco P, Panza E, Ercolano G, et al. ATB-346, a novel hydrogen sulfide-releasing anti-inflammatory drug, induces apoptosis of human melanoma cells and inhibits melanoma development in vivo. Pharmacol Res 2016;114:67-73. [Crossref] [PubMed]
- Yang MY, Lee HT, Chen CM, et al. Celecoxib suppresses the phosphorylation of STAT3 protein and can enhance the radiosensitivity of medulloblastoma-derived cancer stem-like cells. Int J Mol Sci 2014;15:11013-29. [Crossref] [PubMed]
- Jiang X, Zhou J, Giobbie-Hurder A, et al. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 2013;19:598-609. [Crossref] [PubMed]
- Gowrishankar K, Gunatilake D, Gallagher SJ, et al. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLoS One 2015;10:e0123410. [Crossref] [PubMed]
- Kondo A, Yamashita T, Tamura H, et al. Interferon-gamma and tumor necrosis factor-alpha induce an immunoinhibitory molecule, B7-H1, via nuclear factor-kappaB activation in blasts in myelodysplastic syndromes. Blood 2010;116:1124-31. [Crossref] [PubMed]
- Cecil DL, Gad EA, Corulli LR, et al. COX-2 Inhibitors Decrease Expression of PD-L1 in Colon Tumors and Increase the Influx of Type I Tumor-infiltrating Lymphocytes. Cancer Prev Res (Phila) 2022;15:225-31. [Crossref] [PubMed]
- Unger WW, Laban S, Kleijwegt FS, et al. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol 2009;39:3147-59. [Crossref] [PubMed]
- Zhao Y, Jia Y, Shi T, et al. Depression Promotes Hepatocellular Carcinoma Progression through a Glucocorticoids Mediated Up-Regulation of PD-1 Expression in Tumor infiltrating NK Cells. Carcinogenesis 2019; Epub ahead of print. [Crossref] [PubMed]
- Gong YB, Huang YF, Li Y, et al. Experimental study of the mechanism of tolerance induction in dexamethasone-treated dendritic cells. Med Sci Monit 2011;17:BR125-31. [Crossref] [PubMed]
- Tsuda M, Matsumoto K, Inoue H, et al. Expression of B7-H1 and B7-DC on the airway epithelium is enhanced by double-stranded RNA. Biochem Biophys Res Commun 2005;330:263-70. [Crossref] [PubMed]
- Zhang Y, Lv C, Dong Y, et al. Aspirin-targeted PD-L1 in lung cancer growth inhibition. Thorac Cancer 2020;11:1587-93. [Crossref] [PubMed]
- Xiang Z, Zhou Z, Song S, et al. Dexamethasone suppresses immune evasion by inducing GR/STAT3 mediated downregulation of PD-L1 and IDO1 pathways. Oncogene 2021;40:5002-12. [Crossref] [PubMed]
- Botti G, Fratangelo F, Cerrone M, et al. COX-2 expression positively correlates with PD-L1 expression in human melanoma cells. J Transl Med 2017;15:46. [PubMed]
- Shimizu K, Okita R, Saisho S, et al. Impact of COX2 Inhibitor for Regulation of PD-L1 Expression in Non-small Cell Lung Cancer. Anticancer Res 2018;38:4637-44. [Crossref] [PubMed]
- Tang H, Liu Y, Wang C, et al. Inhibition of COX-2 and EGFR by Melafolone Improves Anti-PD-1 Therapy through Vascular Normalization and PD-L1 Downregulation in Lung Cancer. J Pharmacol Exp Ther 2019;368:401-13. [Crossref] [PubMed]
- Liang M, Yang H, Fu J. Nimesulide inhibits IFN-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells by COX-2 and PGE2 independent mechanisms. Cancer Lett 2009;276:47-52. [Crossref] [PubMed]
- Rosenkranz B, Frölich JC. Plasma concentrations and anti-platelet effects after low dose acetylsalicylic acid. Prostaglandins Leukot Med 1985;19:289-300. [Crossref] [PubMed]


