
Infliximab in the treatment of tislelizumab-induced steroid-refractory immune checkpoint inhibitor-related pneumonia: case report and literature review
Introduction
Immune checkpoint inhibitors (ICPs), including programmed cell death-1 (PD-1) inhibitors, PD ligand 1 inhibitors (PD-L1), or cytotoxic T lymphocyte antigen 4 (CTLA-4). ICPs have revolutionized the treatment of lung and many other cancers by disrupting interactions between receptors and ligands in the tumor microenvironment, enabling T cells to recognize and attack cancer cells (1). While blocking the negative regulatory signals of immune cells and enhancing the anti-tumor effect of T cells, ICPs may also abnormally enhance normal immune responses, resulting in the loss of immune tolerance to self-antigens and excessive activation of autoimmunity, manifested as a series of immune-related adverse events (irAEs) (1). Of these irAEs, checkpoint inhibitor-related pneumonia (CIP) occurs in 5–10% of lung cancer patients and can be fatal when it occurs (2-4). Published consensus guidelines can aid in the clinical diagnosis and management of irAEs, but insufficient evidence exists for infliximab in steroid-refractory CIP (3,5). Here, we report a patient with small cell lung cancer (SCLC) suffered from severe CIP after using PD-1 inhibitor combined with chemotherapy and radiotherapy, and review the relevant literature to improve the rational use of infliximab for the treatment of steroid-refractory CIP. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1162/rc).
Case presentation
A 67-year-old male patient was diagnosed with right upper lung small cell lung cancer (T2N2M0 IIIa, limited stage) due to cough and sputum bloody, and received tislelizumab 200 mg D1, irinotecan 240 mg D1, and nedaplatin 110 mg D1 After 2 cycles of treatment (D1, D21), we performed a 4-month follow-up and recorded the imaging changes of the patient’s chest (Figure 1). The curative effect was stable after the second cycle (Figure 1A-1D). Then 30 times of lung radiotherapy (tumour does DT60Gy/30fx) were performed. Cough, expectoration, blood in sputum, and shortness of breath occurred during radiotherapy. Our repeat chest CT showed multiple ground-glass opacities in the upper and lower fields of the right lung, and thickened interlobular septa. Immune-related pneumonia was considered, and radiation pneumonitis was not ruled out (Figure 1E-1H). Prednisone 10 mg orally once a day was given, which had a certain relieving effect. Subsequently, a similar chemotherapy regimen was given in the third cycle, but the immunotherapy was stopped.
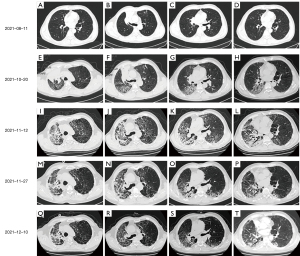
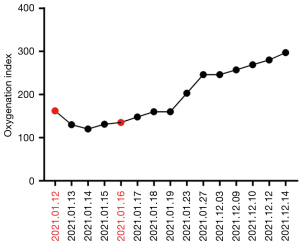
Four and a half months after the first immunotherapy, the patient was admitted to our respiratory medicine unit with rapidly worsening fever and dyspnea. The oxygenation index value for arterial blood gas analysis was 162 mmHg. Blood routine examination showed that the red blood cell (RBC) was 6.26×109/L, the hemoglobin (HGB) was 86 g/L, the platelet (PLT) was 322×109/L, and the neutrophil percentage (Neu%) was 77.10%. Other blood tests showed that procalcitonin (PCT) was 0.75 ng/L, interleukin-6 (IL-6) was 55.60 ng/L, high-sensitivity C-reactive protein (hsCRP) was 131.00 mg/L, and tumor necrosis factor (TNF-α) was 15.90 ng/L (normal range, 0–8 ng/L), IL-1β of 21.9 pg/mL (normal range, 0–5 pg/mL), and IL-8 and IL-10 in the normal range, and five items of liver function, kidney function, and thyroid function were not abnormal. Chest CT showed extensive ground-glass opacities and interlobular septal thickening in both lungs, accounting for approximately 75% of the lung fields (Figure 1I-1L). Combined with the patient’s medical history, laboratory tests, and chest CT, the clinical diagnosis was severe CIP (grade 4), and continuous high-flow humidified oxygen therapy was performed with intravenous injection (IVIG) of high-dose methylprednisolone sodium succinate (240 mg 4 mg/kg qd, continuous 3 days, then tapered) in combination with gamma globulin (0.4 g/kg, 5 days). The patient’s fever improved, but dyspnea and oxygenation index worsened for 3 consecutive days (Figure 2). Therefore, infliximab (5 mg/kg) was added on day 4, and methylprednisolone sodium succinate was started to down-regulate (20% reduction every 10 days). The patient’s dyspnea gradually improved, and the oxygenation index gradually increased from day 5. Our repeat chest CT on the day 12 after infliximab treatment showed obvious absorption of bilateral lung lesions (Figure 1M-1P), and a second dose of infliximab (5 mg/kg) was given. We review chest CT after two doses of infliximab showed further absorption in both lungs (Figure 1Q-1T), and the patient was discharged smoothly. During hospitalization, G test (1,3-β-D glucan test), GM test (galactomannan test), cryptococcal antigen, blood culture, and sputum culture were all negative, but moxifloxacin, meropenem, and voriconazole were administered empirically to control infection.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The overall incidence of CIP is about 0–10% (6), and multiple studies have reported that PD-1 monoclonal antibody has a higher risk of CIP than PD-L1 monoclonal antibody (7). A meta-analysis of 20 studies on PD-1 inhibitors reported an overall incidence of 2.7% (8). In the RATIONALE 304 study, the incidence of CIP was 9% in patients receiving tislelizumab combination therapy (9). Currently, the combination regimens of PD-1/PD-L1 inhibitors and chemotherapy or radiotherapy have become a promising treatment strategy for advanced lung cancer, which does not appear to increase the incidence of CIP obviously, compared to PD-1/PD-L1 inhibitors alone (10,11). The clinical symptoms and imaging findings of patients with CIP are nonspecific, and severe CIP has a high mortality rate (3,4,12). Chest CT can show various interstitial pneumonia-like manifestations, such as acute interstitial pneumonia (AIP), acute respiratory distress syndrome (ARDS), opportunistic pneumonia (OP), and nonspecific interstitial pneumonia (NSIP) (13). The diagnosis of CIP should be based on comprehensive factors, including medication history, clinical manifestations, imaging manifestations, exclusion of infectious, cancerous lymphadenitis, and treatment response. This patient developed radiotherapy-side interstitial pneumonitis during radiotherapy, which is 2.5 months after tislelizumab administration, and CIP (grade 2) was considered, but radiation pneumonitis was not completely ruled out. Lung lesions did not worsen after 10 days of oral prednisone (10 mg/day), but rapid progression of respiratory failure occurred after taking half a month, which is 5 months after immunotherapy, chest CT showed bilateral AIP-like changes, we considered irAEs. The key points for us to consider irAE are through our dynamic follow-up chest imaging, the lung lesions of this patient involved all lobes of both lungs, and the distribution of lesions was not limited to the radiation field, showing a multifocal distribution, followed by rapid progressive ground-glass opacity and consolidation in both lungs. Severe CIP (grade 4) was considered, and confirmed by later examination and treatment response. Steroid- refractory CIP was defined as a failure of clinical improvement of CIP after a minimum of 48 hours of high-dose corticosteroids (prednisone 1–2 mg/kg/day or more) to up to 14 days of corticosteroids (7,14). Steroid-refractory CIP is a fatal clinical phenomenon, and its incidence is poorly understood. Balaji et al. estimated the incidence of steroid- refractory CIP among patients referred for multidisciplinary care, at a surprisingly high 18.5% (15). The most common imaging manifestation is diffuse alveolar damage (DAD), and 75% of primary tumors are lung cancer (15).
According to ASCO guidelines, the management of CIP should be based on the grading of immune-related pneumonitis (5). Grade 2 CIP patients, with new-onset symptoms, generally accumulating 25–50% of the lung fields, need to suspend ICPs treatment and take 1–2 mg/kg/day oral prednisone. Grade 3–4 CIP patients, with severe new-onset symptoms require hospitalization, are recommended to permanently stop ICPs and add intravenous methylprednisolone at 1–2 mg/kg/day. If symptoms improve within 48 hours, the dose can be gradually reduced to discontinuation after 4–6 weeks. For steroid-refractory CIP with no improvement in symptoms and blood oxygen saturation within 48 hours, immunosuppressive agents should be added, including infliximab, mycophenolate mofetil or intravenous immunoglobulin. At the same time, preventive or empirical anti-infective treatment is required (5).
Infliximab is a monoclonal anti-TNF-α antibody used to treat various autoimmune diseases, or severe COVID-19 (16). According to ASCO guidelines, infliximab has become a commonly used drug for the treatment of steroid-refractory irAEs during ICP treatment (5,14). There is relatively sufficient evidence for infliximab in the treatment of steroid-refractory colitis (5,14). However, results are ambiguous for infliximab in the treatment of steroid-refractory CIP, also includes myocarditis (16,17). Some data about infliximab in steroid-refractory CIP are positive (18), but others are opposite. Balaji et al. reported that infliximab-treated group had a mortality rate of 100%, but the gamma globulin for IVIG alone group showed relatively good results (15). Our patient developed new symptoms 2.5 months after the first dose of tislelizuma, chest CT showed interstitial pneumonia in both lobes of the right lung. Unfortunately, only 10 mg/day of prednisone was given, a regimen that may have been insufficient and led to subsequent exacerbations of CIP.
After the patient’s condition progressed rapidly, the inflammatory factors were examined with 55.60 ng/L of IL-6, 131.00 mg/L of high-sensitivity C-reactive protein (hsCRP), 15.90 ng/L (0–8 ng/L) of TNF-α, 21.9 pg/mL (0–5 pg/mL) of IL-1β, and normal range of IL-8 and IL-10. After two doses of infliximab treatment, clinical symptoms and imaging was significantly improved. To our knowledge, there are no materials about the relationship between efficacy of infliximab with any inflammatory factors. This case suggests that inflammatory factors, especially TNF-α, may be prospective in predicting the efficacy of infliximab in the treatment of steroid-refractory CIP (19,20), but a large amount of evidence-based evidence is still needed.
The diagnosis and treatment of CIP is a thorny issue facing the current use of ICPs in the treatment of cancer. There are no prospective clinical trials to determine the identification and optimal treatment of CIP from other pneumonitis. Treatment plan relies mostly on the experience of the medical team. This report implies that infliximab should be considered when high-dose glucocorticoids combined with immunoglobulin is ineffective in severe CIP. More research about inflammatory factors, especially TNF-α, is needed in the future to predict suitable patients for infliximab in the treatment of steroid-refractory CIP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1162/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1162/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1162/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dine J, Gordon R, Shames Y, et al. Immune Checkpoint Inhibitors: An Innovation in Immunotherapy for the Treatment and Management of Patients with Cancer. Asia Pac J Oncol Nurs 2017;4:127-35. [Crossref] [PubMed]
- King GT, Sharma P, Davis SL, et al. Immune and autoimmune-related adverse events associated with immune checkpoint inhibitors in cancer therapy. Drugs Today (Barc) 2018;54:103-22. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol Res 2016;4:289-93. [Crossref] [PubMed]
- Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol 2021;39:4073-126. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer 2019;19:558. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. [Crossref] [PubMed]
- Lu S, Wang J, Yu Y, et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J Thorac Oncol 2021;16:1512-22. [Crossref] [PubMed]
- Chen X, Zhang Z, Hou X, et al. Immune-related pneumonitis associated with immune checkpoint inhibitors in lung cancer: a network meta-analysis. J Immunother Cancer 2020;8:e001170. [Crossref] [PubMed]
- Hwang WL, Niemierko A, Hwang KL, et al. Clinical Outcomes in Patients With Metastatic Lung Cancer Treated With PD-1/PD-L1 Inhibitors and Thoracic Radiotherapy. JAMA Oncol 2018;4:253-5. [Crossref] [PubMed]
- Chuzi S, Tavora F, Cruz M, et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res 2017;9:207-13. [Crossref] [PubMed]
- Nishino M, Ramaiya NH, Awad MM, et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res 2016;22:6051-60. [Crossref] [PubMed]
- Thompson JA, Schneider BJ, Brahmer J, et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw 2020;18:230-41. [Crossref] [PubMed]
- Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer 2021;9:e001731. [Crossref] [PubMed]
- Matzen E, Bartels LE, Løgstrup B, et al. Immune checkpoint inhibitor-induced myocarditis in cancer patients: a case report and review of reported cases. Cardiooncology 2021;7:27. [Crossref] [PubMed]
- Zhang RS, Padegimas A, Murphy KM, et al. Treatment of corticosteroid refractory immune checkpoint inhibitor myocarditis with Infliximab: a case series. Cardiooncology 2021;7:13. [Crossref] [PubMed]
- Beattie J, Rizvi H, Fuentes P, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer 2021;9:e001884. [Crossref] [PubMed]
- Ye C, Zhu S, Yuan J. Characterization of Two TNF-Related Subtypes Predicting Infliximab Therapy Responses in Crohn’s Disease. Front Immunol 2022;13:871312. [Crossref] [PubMed]
- Song YJ, Choi IA, Meylan F, et al. Circulating TNF-like protein 1A (TL1A) is elevated early in rheumatoid arthritis and depends on TNF. Arthritis Res Ther 2020;22:106. [Crossref] [PubMed]


