
Neoadjuvant chemotherapy followed by radical vulvectomy for adenoid cystic carcinoma of Bartholin’s gland: a case report
Introduction
As an extremely rare disease, primary Bartholin’s gland carcinoma (BGC) accounts for less than 1% of female genital cancers (1). Histologically, BGC can be divided into different types such as adenocarcinoma, squamous cell carcinoma, adenoid cystic carcinoma (ACC), adenosquamous carcinoma, etc., among which ACC accounts for about 10–15% (2). ACC of the Bartholin’s gland is a slow-growing, locally aggressive tumor characterized by nerve infiltration. Recurrence and distant metastasis often occur in the late stage (2,3). The most common symptom is vulvar mass, followed by genital pain, bleeding, burning and so on (4). Surgery is the most commonly recommended treatment for BGC, and the most common procedures are simple local excision and radical vulvectomy with or without lymph node dissection. Usually, patients receive adjuvant radiation therapy after surgery and chemotherapy for advanced patients if distant metastases occur. As a rare pathological type in BGC, ACC has no consensus in treatment, and most refer to the treatment of squamous cell carcinoma of the vulva and ACC of the head and neck. However, it has not achieved superior efficacy, and its treatment modality still deserves to be explored. We herein report for the first time a case of neoadjuvant chemotherapy followed by radical vulvectomy for ACC of Bartholin’s gland, which has never been reported before. After the patient received neoadjuvant chemotherapy, the extent of the lesion was significantly reduced, and the boundaries of the mass became more apparent, which reduced the difficulty of surgery, thus reducing surgical complications and enabling complete removal of the tumor tissue which was beneficial to the patient’s prognosis. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2634/rc).
Case presentation
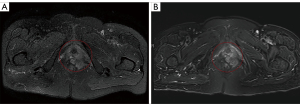
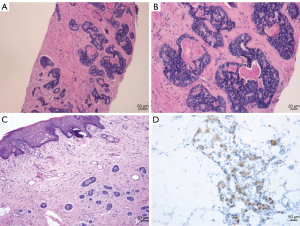
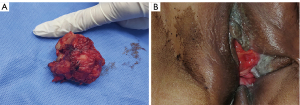
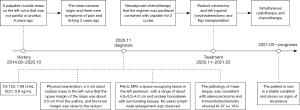
A 69-year-old female presented with a palpable nodular mass on the left vulva 6 years ago that was not painful or pruritus. Three years ago, the mass became more extensive, and there were symptoms of pain and itching. The patient came to Shandong Provincial Hospital for treatment. The serum level of cancer antigen 125 (CA 125) was 7.09 U/mL, and the serum level of squamous cell carcinoma antigen (SCC) was 0.9 ng/mL, both of which were within the normal range. Physical examination revealed a 4 cm-sized nodular mass in the left vulva. The upper margin of the mass was about 0.5 cm from the urethra, and the lower margin was close to the rectum. Pelvic magnetic resonance imaging (MRI) showed a left perineal occupying lesion measuring approximately 4.6 cm × 5.2 cm × 4.0 cm (Figure 1A) with indistinct boundaries to the surrounding tissue. No pelvic lymph node enlargement was observed. Considering the extent of the lesion and its blurred boundaries with the surrounding tissues, it is challenging to perform immediate surgery, and the patient’s quality of life would be reduced after immediate surgery. We decided to conduct two cycles of neoadjuvant chemotherapy before surgery. The chemotherapy regimen was paclitaxel 175 mg/m2 intravenous drip over 3 hours followed by cisplatin 75 mg/m2 intravenous drip and repeated every 3 weeks. The patient received chemotherapy successfully without severe adverse reactions. Physical examination after the neoadjuvant chemotherapy showed that the mass was smaller than before, and the range of motion was increased. Pelvic MRI after the neoadjuvant chemotherapy showed a space-occupying lesion in the left perineum, with a range of about 4.7 cm × 3.8 cm × 3.5 cm (Figure 1B). It was close to the left obturator internal muscle, and fat signals around the rectum existed. The volume of space-occupying lesions has shrunk by about 35%. The surgical methods were radical vulvectomy and left inguinal lymphadenectomy. During the surgery, flap transplantation was performed because of the larger wound surface. The pathology of mass biopsy (Figure 2A,2B) before chemotherapy was consistent with adenocarcinoma, and immunohistochemistry showed Ki-67 (+) 10%. Postoperative pathology confirmed that the size of the tumor was 5×3.5 cm, with extensive invasion into nerve tissues and inguinal lymph node metastasis (Figure 2C). Immunohistochemistry showed CD117 (+), CK (AE1/AE3) (+), CEA (−), S-100 (+), Calponin (−), Ki-67 (10–30%) (Figure 2D). Based on the patient’s clinical manifestations, pathological results of biopsy and surgery, and immunohistochemical results, we finally diagnosed the patient with ACC. The patient was treated with simultaneous radiotherapy and chemotherapy after the operation, and there were no severe complications. The patient was currently in a stable condition at 14 months postoperatively, and the pelvic MRI showed no apparent signs of residual or recurrence. Moreover, a mass about 4 cm in diameter was removed (Figure 3A) during the operation and the vulva recovered well 4 months after the operation (Figure 3B). The timeline of the history, diagnosis, treatment and prognosis of the case is shown in Figure 4. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Although ACC usually occurs in the salivary glands, it can sometimes occur in the respiratory tract, skin, and breasts. In the female genital tract, ACC is more common in the cervix, while ACC originating from the Bartholin’s gland is extremely rare (3,5). To confirm the diagnosis of primary vulvar cancers, we must exclude metastases from these sites. The etiology is still unclear and may be related to persistent infection of the vulva. It has also been reported that pregnancy is one of the risk factors, and hormones may play an important role in its pathogenesis (6). Grossly, the tumor is an ill-defined, dense fibrous infiltrating mass extending into the striated muscle (7). The primary lesion is about 0.5–4 cm in size (1,8). Histologically, neoplastic cells are usually composed of small, uniformly arranged cells that are cord-like or nest-like with a cribriform pattern that shows loose eosinophilic material in the cystic spaces. In addition, mucin or hyalinized basement membrane-like material generally fills the pseudocoel (4,7,9). Immunohistochemically, the tumor cells express the markers of glandular epithelium and myoepithelium, such as keratins, S100, actin, lysozyme, type IV collagen, and p63 (10).
The clinical symptoms and signs of ACC of Bartholin’s gland are unspecific. The average age of the patients is 49 years, ranging from 25 to 80 years (11). The most common symptoms are vulvar mass, followed by pain, pruritus, bleeding, burning, dyspareunia, etc. Most of the lesions are unilateral, and bilateral lesions are rare (4,12). Symptoms such as pain and itching may be due to perineural infiltration, a characteristic microscopic feature of ACC. Therefore, many patients often feel genital itching or burning pain before the appearance of a palpable vulvar mass (13). This disease is easily misdiagnosed as a Bartholin’s gland cyst. If the patient is treated as a Bartholin’s gland cyst, tumor cell dissemination may occur (3). However, the study of Frable et al. (14) proved that fine-needle aspiration biopsy could be used to provide a definitive preoperative diagnosis. When a Bartholin’s gland mass appears on women over 40 years old, we should be alert to the possibility of cancer. Biopsies should be performed first to rule out potential cancer if any solid lesion or enlargement of the Bartholin’s gland is present (12,15).
Because of the rarity of these lesions and the lack of sufficient clinical understanding, there is no consensus on optimal treatment at present, and individual therapy should be implemented according to the situation of different patients. Surgery is currently recommended for most patients in the early stages of the disease. The most common surgery is simple local excision and radical vulvectomy, with or without lymphadenectomy. For the small, unilateral lesion which is not close to the mid-line, simple local excision with a negative margin is feasible. However, radical vulvectomy is required if the lesion is extensive or near the mid-line (10). In order to obtain a clean surgical margin, the resection scope must include removing a portion of the total thickness from the vulva surface to the urogenital diaphragm. During the surgery, plastic reconstruction can restore anatomical integrity if there is a significant defect (8). In order to decrease the risk of local recurrence, the surgical margin is required to be at least 1 cm from the tumor without affecting the function of nearby organs (16). However, the role of margin status in the prognosis of ACC remains controversial. In a study by Yang et al. (5), among the 45 patients, the recurrence rate of the positive and negative margin groups was similar, which is approximately 52%. Therefore, they suggested that the state of the margins may not be as significant as most previous authors have thought. However, this may be because half of the patients with positive margins received adjuvant radiotherapy after surgery. Patients often have recurrence and metastasis for an extended period after treatment, which results in many patients not being followed up for a sufficient length of time. Therefore, the importance of assessing the status of the margin and initial treatment is limited (5). Lymph node metastasis is not very common in ACC of the Bartholin’s gland (17). This tumor generally has distant metastasis before lymph node metastasis (2,15). Distant metastasis usually occurs lately after initial treatment and is hematogenous mainly. The most common sites of metastases are the lung and bone, followed by the liver, kidney, and brain (6,10). Therefore, whether to remove the lymph nodes and the extent of lymph node removal is still controversial. Lymphadenectomy provides two benefits, one is to obtain data on the incidence of local metastases, and the other is to provide prognostic information (17). At the same time, approximately 60% of women who performed lymphadenectomy would have negative lymph nodes histologically. Instead of benefiting from it, patients are at risk of infection, lymphedema, and leg pain (4). If inguinal lymph node dissection is performed, it may be limited to ipsilateral lymph node dissection because no contralateral lymph node involvement has been reported to date (1). The study of Segarra Vidal et al. (15) has considered the feasibility of sentinel lymph node resection in patients with ACC, but there is no evidence to support its benefits. It is difficult to obtain clear surgical margins in patients with advanced disease due to tumor invasion of adjacent organs. Surgery to get negative margins will lead to more complications, and patients will have a poor quality of life. Therefore, radiotherapy and chemotherapy are recommended rather than surgery.
Patients with ACC of Bartholin’s gland usually have a good prognosis. The overall survival rate of this tumor may be mainly affected by the tumor stage and the status of the incision margin. Abrao et al. (9) have reported that patients with negative margins have a longer survival time than patients with positive margins, about 15–30 years. However, the survival time of patients with positive margins was less than 7 years. According to the study of Rosenberg et al. and Copeland et al., adjuvant radiotherapy reduced the possibility of local recurrence in patients with positive margins. Still, it cannot prevent distant metastasis (13,18). Therefore, adjuvant radiotherapy should be considered for patients with positive surgical margins. However, it has also been noted that ACC of Bartholin’s gland is less sensitive to radiotherapy (7). In advanced patients, immediate surgery has a significant chance of causing damage to midline structures such as the anus, urethra, and vagina, resulting in incomplete removal of the tumor and some postoperative complications. On the other hand, neoadjuvant chemotherapy aims to shrink tumors and transform inoperable tumors into radically resectable ones, thus improving the quality of life of patients. In addition, a study of Mousa has shown that neoadjuvant chemotherapy reduces the risk of significant recurrence (19). There is no consensus on chemotherapy for ACC of Bartholin’s gland, and no large sample trials have been conducted for this rare malignancy. Chemotherapy for this tumor is mainly based on existing standards for ACCs of the head and neck and vulvar squamous cancers. Adriamycin, 5-fluorouracil, and cisplatin have been proved to be helpful in the treatment of ACC in the head and neck (19,20). The preferred regimen of chemotherapy is platinum-based chemotherapy. However, the application of the above drugs in the treatment of ACC of Bartholin’s gland needs to be evaluated in more clinical trials. When the tumor is more extensive, surgery after neoadjuvant chemotherapy may be considered, avoiding complications caused by too extensive surgery (4). A Pelvic MRI of our patient showed the lesion is closely related to the surrounding tissues and is relatively fixed. So we decided to conduct neoadjuvant chemotherapy before surgery. After two cycles of chemotherapy, physical examination before the operation showed that the mass was smaller than before, and the range of motion was increased. As well as the pelvic MRI showed that the size of the tumor decreased by about 35%. It has been confirmed that our neoadjuvant chemotherapy regimen may affect ACC of Bartholin’s gland, which supports the following research on the treatment of ACC of Bartholin’s gland.
Due to the rarity of this disease, there are no available reports of neoadjuvant chemotherapy for ACC of the Bartholin’s gland. Although our case showed that neoadjuvant chemotherapy reduced the difficulty of surgery and favored the prognosis for this patient, a single case is not enough to provide valid evidence to support its availability, and more validation is needed. In addition, our patient had a short follow-up period. Despite the significant short-term efficacy, long-term follow-up is necessary to clarify that neoadjuvant chemotherapy benefits the patient’s prognosis.
In conclusion, ACC of Bartholin’s gland is an extremely rare malignancy. The appropriate treatment has not been resolved, and the effectiveness of radiation and chemotherapy has not been established. Based on our patient’s treatment, we speculate that preoperative neoadjuvant chemotherapy may be better for patients with extensive and fixed lesions. Surgery after neoadjuvant chemotherapy may be a feasible and beneficial method for treating early extensive lesions.
Acknowledgments
We thank the Department of Pathology and the operating room of Shandong Provincial Hospital for their selfless help with this article.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2634/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2634/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Woida FM, Ribeiro-Silva A. Adenoid cystic carcinoma of the Bartholin gland: an overview. Arch Pathol Lab Med 2007;131:796-8. [Crossref] [PubMed]
- Sahincioğlu O, Berker B, Güngör M, et al. Adenoid cystic carcinoma of the Bartholin's gland: a rare tumor unmarked by persistent vulvar pain in a postmenopausal woman. Arch Gynecol Obstet 2008;278:473-6. [Crossref] [PubMed]
- Hsu ST, Wang RC, Lu CH, et al. Report of two cases of adenoid cystic carcinoma of Bartholin's gland and review of literature. Taiwan J Obstet Gynecol 2013;52:113-6. [Crossref] [PubMed]
- Di Donato V, Casorelli A, Bardhi E, et al. Bartholin gland cancer. Crit Rev Oncol Hematol 2017;117:1-11. [Crossref] [PubMed]
- Yang SY, Lee JW, Kim WS, et al. Adenoid cystic carcinoma of the Bartholin's gland: report of two cases and review of the literature. Gynecol Oncol 2006;100:422-5. [Crossref] [PubMed]
- Shahabi S, Nathan LM, Chanana C, et al. Liver metastasis in a case of adenoid cystic carcinoma of the Bartholin's gland: a rare presentation. Arch Gynecol Obstet 2009;279:747-50. [Crossref] [PubMed]
- Bernstein SG, Voet RL, Lifshitz S, et al. Adenoid cystic carcinoma of Bartholin's gland. Case report and review of the literature. Am J Obstet Gynecol 1983;147:385-90. [Crossref] [PubMed]
- Barcellini A, Gadducci A, Laliscia C, et al. Adenoid Cystic Carcinoma of Bartholin's Gland: What Is the Best Approach? Oncology 2020;98:513-9. [Crossref] [PubMed]
- Abrao FS, Marques AF, Marziona F, et al. Adenoid cystic carcinoma of Bartholin's gland: review of the literature and report of two cases. J Surg Oncol 1985;30:132-7. [Crossref] [PubMed]
- Chang Y, Wu W, Chen H. Adenoid cystic carcinoma of the Bartholin's gland: a case report and literature review. J Int Med Res 2020;48:300060519863540. [Crossref] [PubMed]
- Hwang TL, Hung YC, Chang HW. Adenoid cystic carcinoma of Bartholin's gland. Taiwan J Obstet Gynecol 2012;51:119-20. [Crossref] [PubMed]
- Bhalwal AB, Nick AM, Dos Reis R, et al. Carcinoma of the Bartholin Gland: A Review of 33 Cases. Int J Gynecol Cancer 2016;26:785-9. [Crossref] [PubMed]
- Rosenberg P, Simonsen E, Risberg B. Adenoid cystic carcinoma of Bartholin's gland: a report of five new cases treated with surgery and radiotherapy. Gynecol Oncol 1989;34:145-7. [Crossref] [PubMed]
- Frable WJ, Goplerud DR. Adenoid cystic carcinoma of Bartholin's gland diagnosis by aspiration biopsy. Acta Cytol 1975;19:152-3. [PubMed]
- Segarra Vidal B, Cañete Mota S, Andrade Cadena P, et al. Adenoid cystic carcinoma of the Bartholin's gland. Int J Gynecol Cancer 2021;31:292-8. [Crossref] [PubMed]
- Oonk MHM, Planchamp F, Baldwin P, et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients With Vulvar Cancer. Int J Gynecol Cancer 2017;27:832-7. [Crossref] [PubMed]
- Nasu K, Kawano Y, Takai N, et al. Adenoid cystic carcinoma of Bartholin's Gland. Case report with review of the literature. Gynecol Obstet Invest 2005;59:54-8. [Crossref] [PubMed]
- Copeland LJ, Sneige N, Gershenson DM, et al. Adenoid cystic carcinoma of Bartholin gland. Obstet Gynecol 1986;67:115-20. [Crossref] [PubMed]
- Mousa A, Rahimi K, Warkus T. Neoadjuvant chemoradiotherapy followed by radical vulvectomy for adenoid cystic carcinoma of Bartholin's gland: a case report and review of the literature. Eur J Gynaecol Oncol 2016;37:113-6. [PubMed]
- Colevas AD, Yom SS, Pfister DG, et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw 2018;16:479-90. [Crossref] [PubMed]


