
Transarterial embolization for rare spontaneous subacute intratumoral hemorrhage of hepatic hemangioma: a case report and literature review
Introduction
Hepatic cavernous hemangioma is the most common benign tumor of the liver, and its pathogenesis is unclear (1). Most hepatic hemangiomas are asymptomatic and seldom undergo malignant transformation. They typically need periodic observation during long-term follow-up. Although the reported incidence of spontaneous rupture of hepatic hemangioma is low (1–4%) (2), the mortality is as high as 36–39% (3). Compared to spontaneous rupture hemorrhage, intratumoral hemorrhage of hepatic hemangiomas is even rarer. Globally, only nine cases have been reported since 1993 (4-12) (Table 1), of which only three cases received accurate preoperative diagnosis (5,7,11). Therefore, clinicians lack in-depth understanding of the diagnosis and treatment. Herein, we reported a case of spontaneous subacute intratumoral hemorrhage of hepatic hemangioma, which was initially misdiagnosed as liver abscess. After two transarterial embolization (TAE) sessions, the patient’s condition was stable and the prognosis was good. We also reviewed all reported cases of spontaneous intratumoral hemorrhage of hepatic hemangioma at home and abroad to better understand the easily misdiagnosed complications of this common hepatic tumor. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-837/rc).
Table 1
| Case No. | Year | Gender/age (years) | Cardinal symptom | Size of lesion (cm) | Preoperative diagnosis | Imaging manifestations | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| US | CT/ MRI | ||||||||
| 1 | 1993 (4) | F/29 | Abdominal pain and vomiting | 9 | Intracapsular hemorrhage of the liver caudate lobe: adenoma? FNH? or hemangioma? | Hypodense lesion within liver | MRI: hemorrhage into the mass | TAE | Cured |
| 2 | 2004 (5) | F/56 | Fever and anemia | 8×5 | Spontaneous subacute intratumoral hemorrhage of hepatic hemangioma | – | MRI: a very high-intensity mass on T2WI, and a low-intensity area on T1WI; T1WI showed heterogeneous high-intensity lesion within the tumor and T2WI showed high-intensity lesion with a peripheral low-intensity rim within the tumor | Resection | Cured |
| 3 | 2007 (6) | M/39 | Abdominal pain | Not mentioned | Cystic liver lesion: liver cancer? | – | CT: large hypodense cystic lesions | Resection | Cured |
| 4 | 2012 (7) | F/35 | Abdominal mass | 15×12 | Intracapsular hemorrhage of hepatic hemangioma | – | CT: a high-density mass within the hepatic hemangioma | Resection | Cured |
| 5 | 2015 (8) | F/54 | Abdominal pain | 4.4×2.8 | Intracapsular hemorrhage of cystic liver lesion | A cystic mass within the liver | CT: a high density, non-enhanced lesion within the cyst mass | Resection | Cured |
| 6 | 2017 (9) | F/52 | Fever | 16×12 | Liver abscess | A nonhomogeneous echo area in the right hepatic lobe; the area was unclearly demarcated from the nearby tissues, showed no clear capsule, and contained a few liquefied spots | CT: a mixed densities mass, irregular enhancement during the arterial phase, and no significant changes during the balance and delayed phase | TAE, then resection | Cured |
| 7 | 2018 (10) | F/70 | Fever and abdominal pain | 7.6×7.3 | Liver mass | A hepatic mass suggestive of cavernous hemangioma | Native CT: a round-oval, well-delimited mass | Resection | Cured |
| 8 | 2020 (11) | F/59 | Fever and cough | 10×9 | Hepatic hemangioma with hemorrhage | – | CT: a circular mixed density mass within liver, which had a clear boundary and contained a ring-shaped, strip-like slightly high-density shadow | Resection | Cured |
| 9 | 2021 (12) | M/65 | Abdominal pain | 9 | Hepatic cystic adenoma hemorrhage or hepatic cyst hemorrhage | A giant cystic mass, which was more likely to undergo intracapsular hemorrhaging | MRI: a cystic mass within the liver, with multiple-room and an uneven signal. The lesion showed isointense or hyperintense signals on T1WI and on T2WI. After the contrast material agent’s infusion, an unenhanced lesion was found in the cyst | Resection | Cured |
CT, computed tomography; FNH, focal nodular hyperplasia; MRI, magnetic resonance imaging; T1WI, T1‑weighted images; T2WI, T2‑weighted images; TAE, transarterial embolization; US, ultrasound.
Case presentation
A 54-year-old female was admitted to a local hospital due to “intermittent fever for more than 10 days”. The maximum body temperature was 39.3 ℃, and this was usually observed after 3 pm, and accompanied by chills and fatigue. The patient had no history of viral hepatitis, oral contraceptive use and recent trauma. Ultrasound examination incidentally identified a 3.0 cm hemangioma in the right hepatic lobe eight years ago, and she received no medical treatment. On physical examination, her conjunctiva was pale, and there was no jaundice in her sclera. Her liver was not palpable below the costal margin, with mild right epigastric tenderness and percussion pain in the hepatic area. Ultrasound examination showed an irregular mixed cystic-solid mass in the right hepatic lobe, with anechoic areas and dense spotty echoes. The possibility of liver abscess was considered. Laboratory assessment showed (Table 2): red blood cell count and hemoglobin level were decreased, white blood cell count, C-reactive protein and procalcitonin were increased. Albumin was 32.2 g/L, creatinine was 33.0 µmol/L, the remaining liver and kidney function tests were normal, and tumor markers were negative. Although antibiotics and blood transfusion were administered, the fever and anemia were not resolved. A drainage tube was placed under the guidance of ultrasound. A total of 40 mL bloody liquid was drained out, and no pus was noted. The pathological diagnosis was blood clot. Surgical treatment was recommended, but the patient refused.
Table 2
| Laboratory indexes (unit)/normal range | In local hospital | After first TAE | Before second TAE | After second TAE |
|---|---|---|---|---|
| Red Blood cell (×1012/L)/3.5–5.5 | 2.25 ↓ | 3.60 | 4.29 | 4.39 |
| White blood cell (×109/L)/4.00–10.00 | 16.07 ↑ | 7.72 | 4.39 | 12.84 ↑ |
| Hemoglobin (g/L)/110–160 | 69 ↓ | 107 ↓ | 133 | 131 |
| Platelet (×109/L)/100–300 | 310 ↑ | 279 | 237 | 219 |
| Prothrombin time (sec)/9.0–15.0 | 13.4 | 14.8 | 12.8 | 12.6 |
| International normalized ratio/0.80–1.50 | 1.21 | 1.16 | 1.02 | 0.94 |
| ALT (U/L)/5–40 | 20 | 16 | 20 | 88 ↑ |
| AST (U/L)/8–40 | 19 | 26 | 21 | 27 |
| Albumin (g/L)/35.0–55.0 | 32.2 ↓ | 33.4 ↓ | 45.7 | 37.3 |
| Total bilirubin (μmol/L)/5.1–20.4 | 8.8 | 7.0 | 5.7 | 7.1 |
| Urea (mmol/L)/2.78–7.32 | 3.0 | 2.09 ↓ | 2.79 | 2.11 ↓ |
| Creatinine (μmol/L)/44.6–133.0 | 33.0 ↓ | 30.0 ↓ | 42.0 ↓ | 43 ↓ |
| C-reactive protein (mg/L)/0.068–8.20 | 136.47 ↑ | 136.00 ↑ | – | 39.30 ↑ |
| Procalcitonin (ng/mL)/<0.5 | 2.74 ↑ | <0.5 | >0.5 ↑ | 0.11 |
| Tumor marker (AFP, CA19-9, CEA, etc.) | All negative | – | All negative | – |
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CA19–9, carbohydrate antigen 19–9; CEA, carcinoembryonic antigen; TAE, transarterial embolization.
On magnetic resonance imaging (MRI) performed after admission to our hospital, the lesion in the right hepatic lobe showed hypointense signal on T1‑weighted images (T1WI) and hyperintense signal on T2‑weighted images (T2WI). Some areas within the tumor showed high intensity on T1WI and T2WI. On T2WI, there was mixed intensity areas and peripheral ringlike low intensity band. After contrast administration, the large mass demonstrated enhancement with centripetal progression, and typically behaved as a hemangioma. No enhancement was found in the mixed intensity areas (Figure 1). Based on the findings of radiological and laboratory examinations, the patient was diagnosed with spontaneous subacute intratumoral hemorrhage of giant hepatic hemangioma.
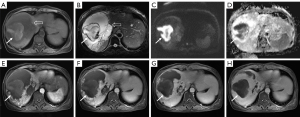
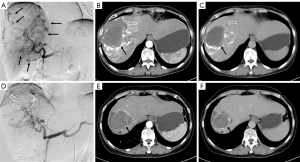
Since the patient refused surgical procedure, interventional treatment was finally adopted after multi-disciplinary team (MDT) discussion involving the gastroenterology department, hepatobiliary surgery department, pathology department, radiology and ultrasonic interventional department. No obvious contrast agent overflow was observed during TAE. Angiography showed that the giant hemangioma in the right hepatic lobe was primarily supplied by the right anterior hepatic artery. The tumor showed “snowy-tree” or “cotton wool” sign, which was consistent with the diagnosis of hepatic hemangioma. Thereafter, 8 mL of pingyangmycin-lipiodol emulsion (containing about 6.4 mg of pingyangmycin) and gelatin sponge particles were injected for embolization. After interventional therapy, the hemoglobin level increased, red blood cells and white blood cells were within normal range, while liver and kidney functions showed no significant changes (Table 2). The patient had no obvious discomfort, and the body temperature decreased to 37.2 ℃. She was also administered symptomatic treatment such as anti-infection and liver protection. The patient recovered well and was discharged uneventfully one week after interventional therapy.
Three months after initial intervention, the patient's laboratory indexes returned to normal (Table 2). Follow-up enhanced computed tomography (CT) showed that the lesion was significantly reduced. We considered another TAE in order to further reduce the size of the lesion and relieve patient’s anxiety. During the second TAE, 6 mL of pingyangmycin-lipiodol emulsion and gelatin sponge particles were injected into the feeding artery branch of the hepatic hemangioma until the tumor staining basically disappeared. Postoperatively, the patient had a slight increase in ALT (88 U/L), right upper abdominal pain and vomiting. She was given symptomatic treatment to relieve pain, stop vomiting and protect the liver, and piperacillin tazobactam for anti-infection. Six days after intervention, the patient’s condition was stable and she was discharged. After 46 months of follow-up, the hepatic hemangioma reduced from 13.4 cm × 11.6 cm to 5.7 cm × 5.4 cm, and the intratumoral hematoma reduced from 6.7 cm × 6.4 cm to 4.4 cm × 4.1 cm (Figure 2). No fever and anemia occurred postoperatively. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Hepatic hemangioma is the most common benign tumor of the liver. Autopsy data have shown that its frequency is 0.4–7.3% (1,13,14). Mocchegiani et al. (15) reviewed the data of more than 83,000 patients who underwent abdominal CT and/or MRI, and found that the prevalence of hepatic hemangioma was 2.5%. The pathogenesis of hepatic hemangioma remains unclear, and some scholars believe that it may be related to abnormal vasculogenesis and angiogenesis (16). Hepatic hemangioma is slow growing and the disease course lasts for several years. Hepatic hemangioma with diameter <4 cm is typically asymptomatic and easily ignored. It is discovered incidentally by imaging examination. A hemangioma with a diameter >4 cm is considered as giant hepatic hemangioma (17), which may cause symptoms (18-20) such as abdominal discomfort and other fatal complications including coagulation dysfunction, etc. In addition to Karabakh-Merritt syndrome, rupture and bleeding are also critical complications of hepatic hemangioma. The typical symptoms present with sudden severe abdominal pain, massive abdominal bleeding or hemorrhagic shock. Since the clinical course of hepatic hemangioma complicated with intratumoral hemorrhage is relatively mild and the symptoms are atypical, clinicians usually lack sufficient understanding of its severity (12). However, fever is a very rare symptom of intratumoral hemorrhage of hepatic hemangioma. Among the previously reported nine cases, three patients developed fever, of which one reported by Hao et al. (9) was also misdiagnosed as liver abscess, after giving antibiotics and blood transfusion to this patient, her fever and anemia did not improve. Liver biopsy specimens showed blood clots and a diagnosis of intratumoral hemorrhage was considered. Angiography showed that the lesion was supplied by the right phrenic artery, with signs of contrast agent overflow. Thus, coil embolization was performed. Fever recurred two days postoperatively; therefore, surgery was performed (9). Shimoji et al. (5) reported a case of subacute intratumoral hemorrhage of hepatic cavernous hemangioma in a patient who had fever and anemia symptoms, and the anti-infection effect was poor. The fever disappeared after hepatectomy, with other infectious factors ruled out by relevant examinations. Another case was also characterized by fever and cough. It was diagnosed as hepatic hemangioma with intratumoral hemorrhage. Finally, the symptoms disappeared after surgical resection (11).
Hepatic hemangioma can be diagnosed by ultrasound, CT, MRI, angiography, positron emission tomography (PET) and other imaging techniques, and the diagnostic accuracy exceeds 90% (21). In ultrasonography, the hemangioma is homogeneously hyperechoic with relatively clear boundary, however, when the mass is accompanied by bleeding, fibrosis or calcification, it could present as hypoechoic (22). In multiphase CT, nodular or patchy contrast-enhanced areas at the edge of the tumor can be observed in the arterial phase and filled into the tumor center over time. In the delayed phase, the entire tumor is uniformly enhanced (23). In addition, when the tumor is complicated with hemorrhage, the hematoma is hyperdense (5,24,25). In contrast, MRI is more valuable because the hematoma has characteristic findings, i.e., high signal on T1WI and T2WI (25). In our case, T2WI showed a circular hypointense structure around the hematoma in the tumor, and T1WI showed that the signal of this structure was hyper and isointense. These results were attributed to hemosiderin deposition around the hematoma, suggesting that the intratumoral hemorrhage was in subacute or chronic stage.
However, for patients with no or unclear history of hepatic hemangioma, it may be difficult to distinguish them from intracapsular hemorrhage of hepatic gallbladder adenoma, hepatic cyst and even liver cancer. Of the nine cases reviewed in this paper, only three cases were diagnosed as intratumoral hemorrhage preoperatively. The options for hepatic hemangioma treatment include surgical resection, TAE, liver transplantation, microwave coagulation, radiofrequency therapy and radiological therapy (26-28). Interventional therapy for hepatic hemangioma has been reported in recent years. At present, it is considered as the first line treatment for multiple and/or giant hemangiomas (29-31). Srivastava et al. (32) reported 18 patients with symptomatic hepatic hemangioma who were treated with interventional therapy, the symptom improvement rate was 100%, and there was no treatment-related death. Li et al. (29) retrospectively evaluated the effectiveness and safety of interventional therapy in 836 patients with hepatic hemangioma, and reported a 100% treatment success rate. During the follow-up period from 12 months to 10 years, 100% of the patients’ symptoms were relieved and no death occurred. Among the nine cases of intratumoral hemorrhage of hepatic hemangioma reviewed in this study, eight cases underwent surgical resection, of which one case underwent surgical resection after TAE without remission of symptoms. Graham et al. (4) reported a case of intratumoral hemorrhage in a pregnant woman who received TAE treatment and achieved good results; and this report is the only other case in which the patient received TAE treatment. During the follow-up of 46 months, the patient’s condition was stable and there were no hemangioma-related symptoms such as fever and anemia. This case also demonstrates that TAE is an effective method for the treatment of intratumoral hemorrhage of hepatic hemangioma.
It is worth mentioning that among the nine previously published reports, this is the only one of embolization using pingyangmycin-lipiodol emulsion during TAE. Pingyangmycin is an anti-tumor antibiotic and a vascular sclerosing agent, which acts by damaging vascular endothelial cells, inhibiting endothelial cell regeneration and producing fibrosis. During TAE, sclerosing emulsion was accumulated in the carvernous sinus of hemangioma through drug-carrying lipiodol, in order to progressively embolize the sinus. Finally, due to the obliteration of sinus cavity, the tumor lacked nutrition, which gradually led to sclerosis and fibrosis. Using pingyangmycin-lipiodol emulsion as embolic agent can significantly reduce the volume of hemangioma and alleviate symptoms (29,30). Pingyangmycin is only available in China, and there are similar studies on the treatment of hepatic hemangioma with bleomycin instead of pingyangmycin in other countries (33,34).
Intratumoral hemorrhage of hepatic hemangioma is very rare, especially in the subacute stage. The symptoms lack specificity, and the clinical reference for diagnosis and treatment is insufficient. Herein, we reported a case of spontaneous subacute intratumoral hemorrhage of hepatic hemangioma, which was mistaken for liver abscess, which is helpful to provide clinicians with a basis for diagnosis and treatment. For patients with previous history of hemangioma, timely MRI can provide higher diagnostic accuracy after they develop symptoms such as fever and anemia. TAE is also a safe and reliable alternative to surgical resection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-837/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-837/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-837/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jia K, Gao Z, Li M, et al. Interventional treatments for hepatic hemangioma: A state-of-the-art review. J Interv Med 2022;5:6-9. [Crossref] [PubMed]
- Tang T, Wang X, Mao Y, et al. Real-world data on the clinicopathological traits and outcomes of hospitalized liver hemangioma patients: a multicenter study. Ann Transl Med 2021;9:1067. [Crossref] [PubMed]
- Hanai S, Kobayashi K, Ito R, et al. Internal Hemorrhaging of Hepatic Hemangioma Presenting with a Fever. Intern Med 2022;61:1475-6. [Crossref] [PubMed]
- Graham E, Cohen AW, Soulen M, et al. Symptomatic liver hemangioma with intra-tumor hemorrhage treated by angiography and embolization during pregnancy. Obstet Gynecol 1993;81:813-6. [PubMed]
- Shimoji K, Shiraishi R, Kuwatsuru A, et al. Spontaneous subacute intratumoral hemorrhage of hepatic cavernous hemangioma. Abdom Imaging 2004;29:443-5. [Crossref] [PubMed]
- Feldman PA, Regev A. Atypical giant hepatic hemangiomas with intratumoral hemorrhage. Clin Gastroenterol Hepatol 2007;5:A24. [Crossref] [PubMed]
- Zhang X, Xu J, Gao Y, et al. Spontaneous hemorrhagic necrosis in hepatic hemangioma: a case and literature review. Chin J Cancer Prev Treatment 2012;19:1350-1.
- Kim JM, Chung WJ, Jang BK, et al. Hemorrhagic hemangioma in the liver: A case report. World J Gastroenterol 2015;21:7326-30. [Crossref] [PubMed]
- Hao F, Yang X, Tian Y, et al. Spontaneous internal hemorrhage of a giant hepatic hemangioma: A case report. Medicine (Baltimore) 2017;96:e8702. [Crossref] [PubMed]
- Dima-Cozma LC, Bitere OR, Pantazescu AN, et al. Cavernous liver hemangioma complicated with spontaneous intratumoral hemorrhage: a case report and literature review. Rom J Morphol Embryol 2018;59:557-61. [PubMed]
- Wang A, Chen H, Huang Z, et al. Spontaneous internal hemorrhage of a giant hepatic hemangioma with infection: a case report and literature review. J Int Med Res 2020;48:300060520976474. [Crossref] [PubMed]
- Yang YG, Chen WF, Mai WH, et al. Spontaneous intracapsular hemorrhage of a giant hepatic cavernous hemangioma: a rare case report and literature review. BMC Gastroenterol 2021;21:84. [Crossref] [PubMed]
- Hoekstra LT, Bieze M, Erdogan D, et al. Management of giant liver hemangiomas: an update. Expert Rev Gastroenterol Hepatol 2013;7:263-8. [Crossref] [PubMed]
- Haring MPD, Cuperus FJC, Duiker EW, et al. Scoping review of clinical practice guidelines on the management of benign liver tumours. BMJ Open Gastroenterol 2021;8:e000592. [Crossref] [PubMed]
- Mocchegiani F, Vincenzi P, Coletta M, et al. Prevalence and clinical outcome of hepatic haemangioma with specific reference to the risk of rupture: A large retrospective cross-sectional study. Dig Liver Dis 2016;48:309-14. [Crossref] [PubMed]
- Giannitrapani L, Soresi M, La Spada E, et al. Sex hormones and risk of liver tumor. Ann N Y Acad Sci 2006;1089:228-36. [Crossref] [PubMed]
- Hajong R. Giant hepatic hemangioma. Indian J Gastroenterol 2013;32:352. [Crossref] [PubMed]
- Nault JC, Blanc JF, Moga L, et al. Non-invasive diagnosis and follow-up of benign liver tumours. Clin Res Hepatol Gastroenterol 2022;46:101765. [Crossref] [PubMed]
- Giavroglou C, Economou H, Ioannidis I. Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol 2003;26:92-6. [Crossref] [PubMed]
- Desai G, Budkule D, Pande P, et al. Pyrexia of Unknown Origin: An Atypical Presentation of Hepatic Hemangioma. Surg J (N Y) 2020;6:e180-4. [Crossref] [PubMed]
- Toro A, Mahfouz AE, Ardiri A, et al. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann Hepatol 2014;13:327-39. [Crossref] [PubMed]
- Ho HY, Wu TH, Yu MC, et al. Surgical management of giant hepatic hemangiomas: complications and review of the literature. Chang Gung Med J 2012;35:70-8. [PubMed]
- Valette PJ, Pilleul F, Crombé-Ternamian A. MDCT of benign liver tumors and metastases. Eur Radiol 2003;13:M31-41. [Crossref] [PubMed]
- Sandulescu LD, Urhut CM, Sandulescu SM, et al. One stop shop approach for the diagnosis of liver hemangioma. World J Hepatol 2021;13:1892-908. [Crossref] [PubMed]
- Vilgrain V, Boulos L, Vullierme MP, et al. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics 2000;20:379-97. [Crossref] [PubMed]
- Özgür Ö, Sindel HT. Giant hepatic hemangioma treatment with transcatheter arterial embolisation and transcatheter arterial chemoembolisation; Comparative results Turk J Med Sci 2021;51:2943-50. [Crossref] [PubMed]
- Dong W, Qiu B, Xu H, et al. Invasive management of symptomatic hepatic hemangioma. Eur J Gastroenterol Hepatol 2019;31:1079-84. [Crossref] [PubMed]
- Prodromidou A, Machairas N, Garoufalia Z, et al. Liver Transplantation for Giant Hepatic Hemangioma: A Systematic Review. Transplant Proc 2019;51:440-2. [Crossref] [PubMed]
- Li Y, Jia Y, Li S, et al. Transarterial Chemoembolization of Giant Liver Haemangioma: A Multi-center Study with 836 Cases. Cell Biochem Biophys 2015;73:469-72. [Crossref] [PubMed]
- Sun JH, Nie CH, Zhang YL, et al. Transcatheter Arterial Embolization Alone for Giant Hepatic Hemangioma. PLoS One 2015;10:e0135158. [Crossref] [PubMed]
- Della Corte A, Marino R, Ratti F, et al. The Two-Step Treatment for Giant Hepatic Hemangiomas. J Clin Med 2021;10:4381. [Crossref] [PubMed]
- Srivastava DN, Gandhi D, Seith A, et al. Transcatheter arterial embolization in the treatment of symptomatic cavernous hemangiomas of the liver: a prospective study. Abdom Imaging 2001;26:510-4. [Crossref] [PubMed]
- Akhlaghpoor S, Torkian P, Golzarian J. Transarterial Bleomycin-Lipiodol Embolization (B/LE) for Symptomatic Giant Hepatic Hemangioma. Cardiovasc Intervent Radiol 2018;41:1674-82. [Crossref] [PubMed]
- Özden İ, Poyanlı A, Önal Y, et al. Superselective Transarterial Chemoembolization as an Alternative to Surgery in Symptomatic/Enlarging Liver Hemangiomas. World J Surg 2017;41:2796-803. [Crossref] [PubMed]


