
A patient with stage IIIB advanced breast cancer who is still alive 24 years after surgery: a case report and remarks on the treatment strategies
Introduction
Breast cancer is a popular malignant disease worldwide. In particular, hormone receptor-positive (HR+) breast cancer is the most common of the subtypes, and many treatment options are now available, including mainly endocrine therapy. However, those prognosis for advanced or recurrent cases remains poor and complete cure is still difficult to achieve. Therefore, it is widely treated by a misinterpretation of the conventional Hortobagyi treatment algorithm, which emphasizes quality of life and delays the start of highly invasive treatments as much as possible (1). Hortobagyi noted that it is important to use all available treatments to obtain maximal control of symptoms, prevent serious complications, and prolong life with minimal disruption of the woman’s lifestyle and quality of life. In the case of recurrent breast cancer, if the only destination is to prolong life without using all of these available treatments and therapies, this inevitably indicates that complete cure is not possible in recurrent breast cancer, and the patients are left with a deep sense of hopelessness. However, with rapid advent of agents possessing novel mechanisms such as cell cycle arrest agents and nuclear signal transduction inhibitors, the prognosis of advanced breast cancer is improving.
Although those treatments have improved the recurrence rate in advanced cases and the prolonged survival period in recurrent cases, the number of cases that can be completely cured is still low.
Recently, improvements of the investigation for immune response systems on the host side have revealed the involvement of immune-related cells in recurrent cancer and their influence on the microenvironment around cancer stem cells. The incorporation of information not only on tumor cells but also on host immune response system may lead to the realization of an innovative treatment. In this report, we present a case in which a patient with stage IIIB advanced cancer had been treated with the aim of achieving a complete cure as far as possible, 24 years ago, when treatment strategies were scarce, as a result, the patient is still good condition and treatable. Through the history of treatments in this case from the past to the present, we would like to remark on the future treatment strategies for recurrent breast cancer. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1363/rc).
Case presentation
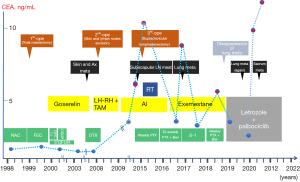
A 52-year-old woman presented to our hospital with a lump in the right breast in March 1998. Physical examination revealed an indistinct borderline mass with a cauliflower-like growing approximately 40 mm from the skin of the right upper lateral mammary gland. Fixation to the pectoralis major muscle was also observed (Figure 1A). Multiple ipsilateral axillary lymph nodes of up to approximately 20 mm were palpated. No medical or family history for the patient was noted. Blood tests revealed no abnormalities, including values for the tumor markers cancer antigen 15-3 and carcinoembryonic antigen (CEA). Initial whole-body computed tomography (CT) findings contained nothing suggestive of distant metastases. A core needle biopsy revealed invasive ductal carcinoma. Although the expressions of the estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor 2 (HER2) and Ki-67 levels were not confirmed, the treatment strategy was decided to perform firstly NAC, based on the diagnosis of advanced breast cancer with cT4N2M0 stage IIIB. In April 1998, we placed a catheter into the internal thoracic artery and epirubicin was trans-arterially administered at a dose of 30 mg 8 times, followed by a single 40 mg intravenous dose (280 mg in total). The treatments induced a marked reduction of the tumor (Figure 1B), and a total mastectomy, combined pectoralis major resection, axillary lymph node dissection and skin grafting were performed in May 1998. Histology examination revealed invasive ductal carcinoma, scirrhous and solid-tubular type and metastases to level I (9/13) and level II (2/3) axillary lymph nodes, but the description of an invasive size was not noted. Immunostaining results were ER-positive, PgR-positive, but both of Ki-67 and HER2 expression were not investigated. Therefore, the pathological stage was pT4cN2aM0 (pathology stage IIIB). Six courses of FEC (5-fluorouracil, epidoxorubicin and cyclophosphamide) followed by oral doxifluridine (600 mg) as post-operative adjuvant chemotherapy was administered from May 1998 in combination with goserelin acetate as endocrine therapy for 5 years, the patient did not receive post-operative radiotherapy. A further course of CPT-11 (80 mg) was administered from March 2000, but discontinued due to the adverse events. Therefore, we administrated 5 courses of cisplatin, 15 mg once for 4 days in April 2001. The local recurrence in the skin and axillary lymph nodes was observed in April 2006, and skin excision and axillary lymph node excision were performed. Histology examination revealed metastatic adenocarcinoma, papillary-tubular type in the axillary lymph nodes and the skin. Immunostaining results were ER-positive, PgR-positive, with negative for HER2 expression, but Ki-67 was not investigated. As adjuvant therapy, docetaxel (DTX) 40 mg tri-weekly for 6 courses, LH-RH agonists and tamoxifen (TAM) was administered from April 2006 and changed to anastrozole in April 2009. No radiotherapy was performed again. The numbness in the patient’s right radial nerve region appeared in February 2015. CT images showed multiple lymph node metastases from the ipsilateral supraclavicular to the axilla (Figure 2A) and small metastatic lesions in lungs (Figure 2B). Therefore, supraclavicular and axillary lymphadenectomy was performed in April 2015. Histology examination revealed invasive ductal carcinoma, solid-tubular type and scirrhous type. Immunostaining results were 70% ER-positive, PgR-negative, and 15% Ki-67-positive, with negative HER2 expression by fluorescence in situ hybridization. In May 2015, radiotherapy (60 Gy in 30 fractions) was administered to the region of supraclavicular lymph nodes followed by 12 courses of weekly paclitaxel (PTX) administration from July to December 2015 and the administration of anastrozole was switched to exemestane in April 2016. However, CT images showed worsening of lung metastases and the enlarged right supraclavicular lymph node in August 2016 (Figure 3A,3B), and the patient was treated with 3 courses of triweekly-PTX and bevacizumab (Bev.). As a result, a significant reduction of pulmonary metastases was confirmed by CT images in October 2016. Therefore, chemotherapy was changed to the administration of oral S-1 (100 mg/day, 2 rests per 4 weeks) as less invasive agents from December 2016. Subsequently we started the administration of oral capecitabine, which resulted in progression disease, so PTX and Bev. was administered for 3 courses from January 2018, having previously achieved a therapeutic response. In December 2017, palbociclib as cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) received marketing approval, the treatment with letrozole and palbociclib was started as combination therapy in July 2018, resulting in almost complete resolution of pulmonary metastases and right supraclavicular lymph node metastasis by August 2018 (Figure 3C,3D). However, CT images again showed a right pulmonary metastasis in April 2019 (Figure 4A) and a small metastatic lesion appeared in the sacrum in August 2020 (Figure 4B). As of November 2021, approximately 15 years after the first recurrence, although multiple bone metastases and a solitary pulmonary metastasis is present (Figure 4C,4D), the patient has been continued the treatment with letrozole and palbociclib and is currently on outpatient monitoring with a performance status (PS)-1. Also, there is no evidence of muscle weakness or neuropathy due to pectoralis major muscle resection at present.
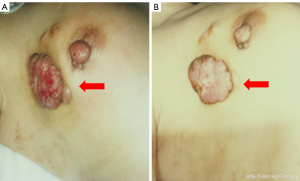
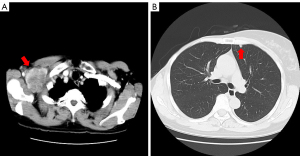
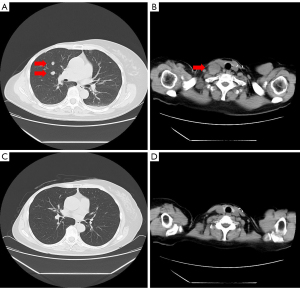
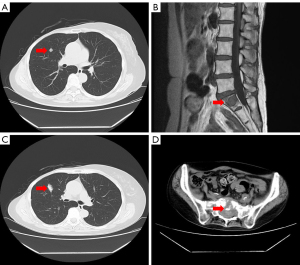
Figure 5 charts the patient’s clinical course from admission to the time of writing.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Even today, the prognosis for metastatic or recurrent breast cancer is extremely poor, with a 10-year survival rate of about 9–11% even with treatment (2), although there are differences depending on the subtypes. At present, the treatment strategies for postmenopausal HR+ HER2− advanced breast cancer are detailed in various clinical guidelines around the world, but 23 years ago in 1998, the concept of subtypes was not even widespread, available agents were limited, and treatment strategies classified according to differentiation of subtype and each stage of disease were not established and widespread. For example, it is widely recognized NAC should be performed as basically initial treatment for advanced cancer, but this treatment strategy followed the NSAP B-18 trial on 1997 (3), which confirmed no difference in overall survival (OS) and disease-free survival (DFS) between pre- and post-operative chemotherapy. Later clinical trials have confirmed that an achieving pathological complete response (pCR) by NAC results in subsequent improvements in prognosis, especially in HER2 enriched type (4).
Recently, with rapid development of the treatment strategies and advent of various agents possessing new mechanism, a survival rate of advanced breast cancer has improved. The data from The Surveillance, Epidemiology, and End Results (SEER) Program shows that the 5-year post-operative survival rate for Stage IIIB breast cancer was 41% in 2001, 20 years ago, and now has improved to about 50%. In particular, the current clinical treatment guidelines for postmenopausal HR+ HER2− advanced breast cancer now describe a various range of options, resulting in somewhat better outcomes compared to other subtypes. However, those prognoses remain poor in highly advanced and recurrent cases. The high incidence of late recurrence has also become a worrying problem in HR+ advanced breast cancer.
Although the definition of long-term survival in recurrent breast cancer is not clear, the cases surviving more than 5–10 years after recurrence are generally considered to be long-term survival cases (5). However, Dr. Patricia Tai states that a certain threshold number of years is required for long-term survival of cancer, especially breast and thyroid cancer, which require longer follow-up than other cancers, and the definition of long-term survival for breast cancer may be different from that of other cancers (6).
Currently, the common initial treatment strategy for HR+ HER2− advanced breast cancer with stage IIIA or higher should be NAC unless distant metastases are present. In this case, trans-arterial chemotherapy via an internal thoracic artery was performed as NAC, but at present, trans-arterial chemotherapy had not been shown an efficacy as it did not improve OS or DFS compared with systemic chemotherapy (7). We would like to mention one thing here about the significance of initial surgery. Currently, in the treatment of advanced breast cancer, the importance of surgery, the classic method of local control, may be often neglected in contrast to the rapidly developing systematic therapy using with rapid advent of agents possessing novel mechanisms. In this case, however, complete resection of the tumor, including the pectoralis major muscle, as the initial treatment provided sufficient local control, which may have enabled long-term disease control. An initial surgical treatment is undoubtedly important in a treatment strategy of complete cure. A total mastectomy, including the surrounding tissue, is certainly an important first step toward a complete cure. This case raises a critical question of whether mastectomy, including the pectoralis major muscle and other surrounding tissues, should be universally denied for the treatment of advanced breast cancer. Subsequently, as post-operative adjuvant therapy, the administration of aromatase inhibitors is commonly chosen for postmenopausal patients, based on the results of ATAC trials (8). However, LH-RH agonists and TAM were administrated in this case, LH-RH agonists should not be commonly used for the post-menopausal patients. Furthermore, we have performed the concurrent use of chemotherapy and endocrine therapy, but the concurrent use of intravenous chemotherapy and endocrine therapy is also not common.
For the treatment of local recurrence, surgical therapy and radiotherapy are commonly chosen for axillary lymph node or intramammary recurrence, but surgical therapy must not be performed for supraclavicular lymph node metastases, radiotherapy and systemic pharmacotherapy are the basic strategy. In addition, chemotherapy had been performed in this case, but currently, endocrine therapy is now widely used as the first choice for HR+ recurrent breast cancer patients based on a misinterpretation of the Hortobagyi treatment algorithm, if there are no life-threatening visceral metastases. The aggressive treatment with anti-HER2 antibody-related agents and immune checkpoint inhibitors (ICIs) is possible for recurrent HER2 enriched type (9) or TN breast cancer (10), while in HR+ HER2− breast cancer, the treatment strategy is more likely to delay the start of chemotherapy and emphasizes quality of life.
As described above, this case has been treated far from the standard treatment guidelines for recurrent breast cancer from a current point of view. Presuming that we treated this case at the present time in 2022, the first step would be NAC using FEC administration followed by DTX administration, then progressing in the order of surgical treatment and postmastectomy radiation therapy. Family history should also confirm the presence of BRCA1/2 mutations. Subsequently, the administration of CDK4/6is with endocrine therapy for two years would be considered as post-operative adjuvant therapy (11). Otherwise, the combined endocrine therapy with oral S-1 (12), or, in elderly patients, the administration of oral UFT for 2 years would be considered (13). As for the first local recurrence, since it was a late recurrence 8 years after surgery and would be considered acquired resistant, so the first step would be the local control with surgical therapy and radiation therapy. Subsequently, if possible, consider changing the AI to selective ER degraders (SERDs) by confirming the presence of ESR1 mutation in the resected specimen or by liquid biopsy, in addition, CDK4/6is would be replaced by unused different CDK4/6is. Moreover, it should be also advisable to investigate BRCA1/2 mutation, programmed cell death ligand-1 (PD-L1) expression, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutation and the presence of microsatellite-instability (MSI) and should consider the administration of the agents with novel mechanisms such as olaparib (14), ICIs, and alpelisib (15).
Approximately 16 years after the first surgery, a systemic recurrence with multiple small pulmonary metastases occurred. However, except in a life-threatening condition, if the endocrine agent used was AI, it should be changed to SERDs, and CDK4/6is should be also changed to unused agents, or a combination therapy with exemestane and everolimus may also have been considered (16).
The treatment strategies described above may be the general treatment strategy at present. However, we may currently in the phase where we had to reconsider the treatment strategies for recurrence and metastatic in HR+ HER2- breast cancer. We consider that it is important to decide whether to follow the conventional treatment bound by a misinterpretation of the Hortobagyi algorithm and avoid highly invasive chemotherapy to prolong life and maintain quality of life without aiming for complete cure, or to adopt a treatment strategy that aims for complete cure from the beginning. The prognosis for patients could vary greatly depending on which treatment is chosen. Although extreme, it may be speculated that if the patient had continued the endocrine therapy alone bound by the conventional treatment policy that emphasizes quality of life without aiming for complete cure, she would not have survived as long. This fact may be the result of continued treatments that aimed to the complete cure, suggesting that the continuation of aggressive treatment can lead to long-term survival.
Recently, with rapid advent of agents possessing novel mechanisms、it may be possible to completely treat and cure for post-menopausal HR+ HER2− breast cancer with systemic recurrence.
Furthermore, we must consider new therapeutic strategies that comprehensively integrate the information not only from tumor cells but also from the host’s immune system. Although we tend to focus on the information of tumor cells, such as tumor infiltrating lymphocytes (TILs) (17), PD-L1, PIK3CA mutation and ESRI mutations, there is no doubt that the information on the host immune response system and the microenvironment around cancer stem cells are also important for the therapeutic strategies. As immune cells involved in the anti-tumor effect on the host side, in addition to cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells which directly attack tumor cells, both dendritic cells (DCs) which recognize tumor-specific antigens and regulatory T cells (Tregs) which suppress anti-tumor immune response are also very important (18). Therefore, the accumulation of the information on expression of proteins and genes such as PD-L1 and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and their expression intensities in tumor cells, peripheral blood lymphocytes, and TILs in metastatic tissues, as well as surface markers of immune-related cells and transition factors such as CD4, 8, 25, 45R, CCR4/6 and Foxp3 may provide a bridge to new treatments (19).
In addition, it has also been proved that cytotoxic anticancer agents significantly impair immune response systems of the patient and progress tumor growth. In view of this, the synergistic anti-tumor effects may be expected if treatment will be possible by combining non-cytotoxic anti-cancer agents that can preserve the function of host immune response system, such as nuclear signal transduction inhibitors, molecular targeting agents, and cell cycle arrest agents, with the treatment which can enhance the functions of CTLs, DCs, and naive memory T cells directly involved in the host immune response system, and antibodies that could inhibit Tregs function. In fact, the latest basic researches have confirmed that CDK4/6is are non-cytotoxic anticancer agents and do not significantly impair the host immune response system, specifically abemaciclib enhances that by suppressing Treg function (20).
A paradigm shift in the treatment strategies, such as occurred in HER2+ breast cancer, may occur in postmenopausal HR+ highly advanced or recurrent breast cancer in the future, and therefore the results of the latest basic research must be kept in mind in clinical practice.
We hope to investigate a comprehensive approach, including the search for biomarkers and gene panel analysis for predicting treatment response in long-term survivors of metastatic or recurrent breast cancer, as well as the analysis of individual immune response states expressed by T cells, Tregs and other immune-related cell functions.
Conclusions
We reported a case of HR+ HER2− advanced breast cancer with stage IIIB at initial diagnosis after various treatments that were available at the time, who is surviving long-term 24 years after the initial operation, and the patient continues to do well despite repeated recurrences and remissions. Now that development of the agents possessing new mechanisms and the ability to investigate the immune response system on the host side, it may be possible to select the treatment aiming for complete cure instead of the conventional treatment strategies bound by a misinterpretation of the Hortobagyi algorithm for recurrent breast cancer.
This report is only one small case report and does not have the power to determine significant changes in the current treatment strategies for recurrent advanced breast cancer. However, we hope that medical professionals involved in the treatment of breast cancer around the world will consider all possibilities to improve the prognosis of current patients with recurrent or metastatic breast cancer, and that new treatment strategies will be obtained through maximum cooperation between basic medical researchers and clinical physicians.
Acknowledgments
The authors express thanks to Yasuhiro Ishino of the Hijirigaoka Hospital Testing Department for processing the artic images.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting Checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1363/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1363/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1363/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hortobagyi GN. Treatment of breast cancer. N Engl J Med 1998;339:974-84. [Crossref] [PubMed]
- Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017;26:809-15. [Crossref] [PubMed]
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778-85. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Soerjomataram I, Louwman MW, Ribot JG, et al. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat 2008;107:309-30. [Crossref] [PubMed]
- Tai P, Yu E, Cserni G, et al. Minimum follow-up time required for the estimation of statistical cure of cancer patients: verification using data from 42 cancer sites in the SEER database. BMC Cancer 2005;5:48. [Crossref] [PubMed]
- Fiorentini G, Tsetis D, Bernardeschi P, et al. First-line intra-arterial chemotherapy (IAC) with epirubicin and mitoxantrone in locally advanced breast cancer. Anticancer Res 2003;23:4339-45. [PubMed]
- Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 2003;98:1802-10. [Crossref] [PubMed]
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [Crossref] [PubMed]
- Adams S, Diéras V, Barrios CH, et al. Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann Oncol 2020;31:582-9. [Crossref] [PubMed]
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 2020;38:3987-98. [Crossref] [PubMed]
- Mukai H, Takashima T, Hozumi Y, et al. Randomized study of taxane versus TS-1 in women with metastatic or recurrent breast cancer (SELECT BC). Jpn J Clin Oncol 2010;40:811-4. [Crossref] [PubMed]
- Kasumi F, Yoshimoto M, Uchino J, et al. Meta-analysis of five studies on tegafur plus uracil (UFT) as post-operative adjuvant chemotherapy for breast cancer. Oncology 2003;64:146-53. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. [Crossref] [PubMed]
- André F, Ciruelos EM, Juric D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol 2021;32:208-17. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol 2016;2:1354-60. [Crossref] [PubMed]
- Law AM, Lim E, Ormandy CJ, et al. The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy. Endocr Relat Cancer 2017;24:R123-44. [Crossref] [PubMed]
- Syed Khaja AS, Toor SM, El Salhat H, et al. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget 2017;8:33159-71. [Crossref] [PubMed]
- Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548:471-5. [Crossref] [PubMed]


