
High expression of ceramide synthase 5 predicts a poor prognosis in gastric cancer
IntroductionOther Section
Gastric cancer is one of the most common malignant tumors worldwide. The global incidence rate and mortality rate are 5th and 4th, respectively. In 2020, more than one million new cases of gastric cancer were diagnosed globally (1). The prognosis of gastric cancer is poor and the 5-year overall survival (OS) rate is only 35.1% (2). Surgical resection is the main method for the treatment of gastric cancer (3). In recent years, increasing strategies were applied for the treatments of gastric cancer, such as chemotherapy, radiotherapy, immunotherapy, and so on (4,5). However, comprehensive treatments for gastric cancer have not improved the OS rates significantly, due to toxicity, side effects, and drug resistance (6). At present, the early screening and diagnosis of gastric cancer mainly rely on imaging, tumor biomarkers, endoscopy, and tissue biopsy (7). Pathological diagnosis by endoscopic biopsy is the gold standard for the diagnosis of gastric cancer, however, it is not widely used for early cancer screening due to its invasive prosperity. Therefore, it is of great importance to search for biomarkers for early diagnosis or targeted treatment for gastric cancer.
Ceramide synthases (CerS) are enzymes that are essential for the de-novo synthesis of ceramides and other sphingolipids. Six types of CerS (CerS1–CerS6) have been found in mammals (8,9). Different ceramides have fatty acyl-coenzyme A with different chain lengths, which implies that they might be involved in the regulation of sphingomyelin metabolism in different types of tissues (10). Ceramides are not only required for the structural components of cellular membranes, but also the signal molecules that trigger cell death and tumor suppression (11,12). Many studies have confirmed that CerS plays an important role in the development of human cancers, such as colon cancer, lung cancer, breast cancer, and so on (13-15).
Ceramide synthase 5 (CerS5), as a member of the CerS family, has been confirmed to be involved in the regulation of the occurrence and development of several types of human cancers. For example, the mRNA expression levels of CerS5 were shown to be upregulated in cancer tissues of patients with colorectal cancer (CRC) (16). The increased CerS5 expression is negatively correlated with the poor prognosis of CRC patients (17). Jiang et al. reported that the levels of CerS5 mRNA and protein in human neuroglioma tissues were significantly higher than those in normal nervous ganglion tissues (18). And the overexpression of CerS5 has been reported to trigger cellular apoptosis via the promotion of ceramide up-regulation following hypoxia or reoxygenation (19). On the contrary, the knockdown of CerS5 by using CerS5-specific shRNA inhibited autophagy and increased the drug sensitivity of HCT116 cells to chemotherapeutics, such as oxaliplatin and 5-FU (20). However, the relevance of CerS5 to gastric cancer is remaining to be addressed.
In this study, we included gastric cancer patients both from our institute and online databases and determined the correlation of CerS5 levels with clinicopathological characteristics of gastric cancer patients from different populations. We aimed to elucidate the differential expression of CerS5 in gastric cancer patients, and uncover the role of CerS5 in the prediction of the prognosis of gastric cancer. We present the following article in accordance with the REMARK reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1348/rc).
MethodsOther Section
Patients
This study included 150 patients who underwent radical gastrectomy in the Zhejiang Cancer Hospital (ZJCH) from January 2013 to December 2017. Inclusion criteria: (I) gastric cancer was definitely diagnosed by postoperative pathology; (II) the patient’s medical records are relatively complete; (III) no comprehensive anti-tumor treatments such as radiotherapy, chemotherapy, targeted therapy, or immunotherapy were performed before operation; (IV) OS follow-up data were complete. Exclusion criteria: (I) patients with any other types of malignant tumors; (II) patients with metastasis from other malignant tumors. We retrospectively collected the medical record data of these patients, including demographic and clinicopathological characteristics, and obtained the OS via telephone follow-up. The final follow-up time was August 2021. OS was defined as the duration from initial surgery to death or the last follow-up.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee board of the Zhejiang Cancer Hospital (IRB-2021-431) and individual consent for this retrospective analysis was waived.
Immunohistochemistry (IHC)
The tissue samples were fixed in formalin and embedded in paraffin. After careful selection by two independent pathologists, representative gastric cancer tissues, paracancerous tissues, and metastatic lymph nodes were collected to construct tissue microarray and subjected to IHC staining. The slices were incubated at 56–60 ℃ for 15 min, performed two changes of xylene, rehydrated with ethanol, 90% ethanol, 80% ethanol, rinsed in gently running tap water, and washed with 1 × phosphate-buffered saline (PBS). Subsequently, the slices were incubated three times with water in a microwave oven for 5 min, cooled slowly at room temperature, incubated with 3% hydrogen peroxide for 5 min, blocked with primary antibody (anti-CerS5 1:500) at 4 ℃ overnight, washed with 1×PBS three times for 5 min, blocked with secondary antibody (goat anti-rabbit IgG H&L, 1:1,000) at room temperature for 30 min, washed with 1×PBS three times for 5 min. Then, the rabbit-specific HRP/DAB (ABC) Detection IHC Kit (Abcam, ab64261) was used for the detection of DAB, and the nucleus was stained with hematoxylin. Finally, the tissue microarray was dehydrated and sealed with neutral gel.
Evaluation of CerS5 staining
The results of the immunohistochemistry assay were interpreted by two senior pathologists. The expression intensity of CerS5 was evaluated by the H-score system. The formula of the H scoring system is as follows: H score = (∑ IS × AP). IS value represents the staining intensity. No staining is 0 points, weak staining is 1 point, and moderate staining is 2 points. AP value represents the percentage of positive staining cells. 0% is 0 points, 1–25% is 1 point, 26–50% is 2 points, 51–75% is 3 points, 76–100% is 4 points. The median H-score of CerS5 is 4 points, which was set as the cut-off value, and the patients with different levels of CerS5 were divided into a high expression group and a low expression group.
The public tumor databases
RNA-seq data of patients with gastric cancer were downloaded from The Cancer Genome Atlas (TCGA) and Asian Cancer Research Group (ACRG) (21) databases, including 405 cases from TCGA and 246 cases from ACRG. We compared CerS5 levels in gastric cancer tissues and paracancerous tissues. According to the corresponding clinicopathological information, the relevance of CerS5 levels to the survival time of gastric cancer patients was determined by the Kaplan-Meier method.
Statistical analysis
SPSS 25.0 software was used for statistical analysis. Chi-square test, corrected Chi-square test, and Fisher’s exact test was used to analyze the correlation between the expression level of CerS5 and clinicopathological features. Kaplan-Meier method was used to draw the survival curve. Univariate and multivariate Cox regression was used to analyze the independent factors affecting the prognosis of patients with gastric cancer. The hazard ratio (HR) and 95% CI were calculated. P<0.05 represents statistical significance.
ResultsOther Section
Clinicopathological features of 150 gastric cancer patients
The median age of gastric cancer patients included in the study was 61 years old, including 106 males (70.7%) and 44 females (29.3%). In terms of tumor location and distribution, patients with distal gastric cancer were the majority, accounting for 61.3%. In terms of TNM stages, the majority of patients in the study were stage III, accounting for 78.7%, and stage II and IV patients accounted for 12.0% and 9.3%, respectively. Among the 150 patients, pathology analysis showed that the degree of differentiation was mainly low differentiation and median-low differentiation, accounting for 44% and 34.7%, respectively. All patients were followed up regularly. The last follow-up time was August 2021. During the follow-up period, 81 patients died. More specific clinicopathological features were shown at https://cdn.amegroups.cn/static/public/tcr-22-1348-1.xlsx and in Table S1.
CerS5 levels increased in tumor tissues of gastric cancer patients
By using immunohistochemistry, we found that CerS5 is expressed in 118 of 150 patients with gastric cancer, accounting for 78.7%, and 32 of 150 patients with negative CerS5 staining account for 21.3% (Figure 1 and Table S2). According to the H-score evaluation standard, the median H-score of CerS5 is 4 points, which was set as the cut-off value. The patients with H-score ≤4 points were categorized as the CerS5 low expression group, and the patients with H-score >4 points were categorized as the CerS5 high expression group. High levels of CerS5 exist in the tumor tissues of 50/150 (33.3%) patients, while only in paracancerous tissues of 24/150 (16.0%) patients (Figure 2A and Table S3). These data indicated that the levels of CerS5 in tumor tissues are significantly higher than those in paracancerous tissues of gastric cancer patients.
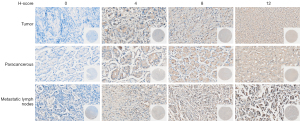
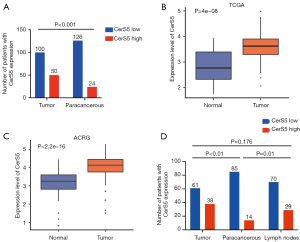
To investigate the role of CerS5 in gastric cancer patients from different populations, we downloaded the RNA-seq data of CerS5 from TCGA and ACRG databases (Table S4, https://cdn.amegroups.cn/static/public/tcr-22-1348-2.xls, https://cdn.amegroups.cn/static/public/tcr-22-1348-3.xls). In terms of proportion and clinical characteristics, TCGA is mainly based on the European population, while ACRG is based on the Asian population. We collected 373 tumor tissues and 32 normal tissues from TCGA, and collected 122 tumor tissues and 123 normal tissues from ACRG, respectively. The results showed that CerS5 is overexpressed in cancer tissues compared to normal tissues in both TCGA and ACRG (Figure 2B,2C), implying that CerS5 is universally upregulated in gastric cancer patients, regardless of the populations.
CerS5 levels increased in metastatic lymph nodes of gastric cancer patients
To test if the expression level of CerS5 varies between cancer tissue and corresponding metastatic lymph node tissue, the pairs of tissues from the same patient were collected and subjected to IHC staining. We found that perigastric lymph node metastasis exists in 99/150 gastric cancer patients, indicating that the metastasis rate is around 66.0%. Moreover, 29 cases of 99 patients (29.3%) with perigastric lymph node metastasis had high expression of CerS5, and 70 cases (70.7%) had low expression of CerS5. Among the paired 99 gastric cancer tissue samples, 38 cases had high expression of CerS5 (38.4%), and 61 cases (61.6%) had low expression of CerS5. Nevertheless, in the paired 99 paracancerous tissue samples, 14 cases had high expression of CerS5 (14.1%), and 85 cases (85.9%) had low expression of CerS5 (Table 1). The results showed that the expression of CerS5 in metastatic lymph node tissues is significantly higher than that in paired paracancerous tissues (P=0.01), while there is no significant differential expression of CerS5 between gastric cancer tissues and metastatic lymph node tissues (P=0.176). These data suggested that CerS5 is overexpressed in both cancer tissues and metastatic lymph nodes tissues, compared with paracancerous tissues of gastric cancer patients (Figure 2D).
Table 1
| Parameters | N | CerS5 expression | Positive rate | χ2 | P value | |
|---|---|---|---|---|---|---|
| High | Low | |||||
| Tumor | 99 | 38 | 61 | 38.4% | 15.022 | <0.001** |
| Paracancerous tissue | 99 | 14 | 85 | 14.1% | ||
| Lymph nodes | 99 | 29 | 70 | 29.3% | 6.684 | 0.010* |
| Paracancerous tissue | 99 | 14 | 85 | 14.1% | ||
| Tumor | 99 | 38 | 61 | 38.4% | 1.827 | 0.176 |
| Lymph nodes | 99 | 29 | 70 | 29.3% | ||
*, P<0.05; **, P<0.001.
The relevance of CerS5 levels to clinicopathological features in gastric cancer
To investigate the relevance of CerS5 to clinicopathological features of gastric cancer, the retrospective clinicopathological data were collected and subjected to statistical analysis by using several statistical methods, including Chi-Square Test, corrected Chi-Square Test, and Fishers’ Exact Test. As shown in Table 2, the main tumor locations of gastric cancer patients with low levels of CerS5 were distal gastric cancer (66/96, 68.8%), however, the main tumor locations of patients with high levels of CerS5 were proximal (23/54, 42.6%) and distal (26/54, 48.1%) gastric cancer. The correlation of CerS5 levels with tumor location is of statistical significance (P=0.045), suggesting that CerS5 is involved in the distribution of gastric cancer.
Table 2
| Parameters | CerS5 expression | Total | χ2 | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Age (year) | |||||
| ≤61 | 53 | 24 | 77 | 1.603 | 0.206 |
| >61 | 43 | 30 | 73 | ||
| Gender | |||||
| Male | 68 | 38 | 106 | 0.004 | 0.952 |
| Female | 28 | 16 | 44 | ||
| Family history (GC) | |||||
| No | 84 | 43 | 127 | 1.649 | 0.199 |
| Yes | 12 | 11 | 23 | ||
| Smoking history | |||||
| No | 59 | 38 | 97 | 1.201 | 0.273 |
| Yes | 37 | 16 | 53 | ||
| Drinking history | |||||
| No | 72 | 41 | 113 | 0.016 | 0.900 |
| Yes | 24 | 13 | 37 | ||
| Weight loss | |||||
| No | 60 | 36 | 96 | 0.260 | 0.610 |
| Yes | 36 | 18 | 54 | ||
| Tumor location | |||||
| Proximal | 25 | 23 | 48 | 6.201 | 0.045* |
| Distal | 66 | 26 | 92 | ||
| Total | 5 | 5 | 10 | ||
| Lauren classification | |||||
| Intestinal | 49 | 24 | 73 | 0.706 | 0.703 |
| Diffuse | 31 | 20 | 51 | ||
| Mixed | 15 | 10 | 25 | ||
| Unknown | 1 | 0 | 1 | ||
| Tumor size (cm) | |||||
| <5 | 31 | 15 | 46 | 0.345 | 0.557 |
| ≥5 | 63 | 38 | 101 | ||
| Unknown | 2 | 1 | 3 | ||
| Grade of differentiation | |||||
| Poor | 43 | 23 | 66 | 0.529 | 0.768 |
| Moderate-poor | 34 | 18 | 52 | ||
| Moderate | 15 | 11 | 26 | ||
| T stage | |||||
| T1/2 | 6 | 0 | 6 | 2.076 | 0.150 |
| T3/4 | 90 | 54 | 144 | ||
| N stage | |||||
| N0/1 | 31 | 13 | 44 | 1.126 | 0.289 |
| N2/3 | 65 | 41 | 106 | ||
| M stage | |||||
| M0 | 90 | 46 | 136 | 2.996 | 0.083 |
| M1 | 6 | 8 | 14 | ||
| TNM stage | |||||
| II | 15 | 3 | 18 | 5.646 | 0.059 |
| III | 75 | 43 | 118 | ||
| IV | 6 | 8 | 14 | ||
| Nerve invasion | |||||
| No | 28 | 12 | 40 | 0.852 | 0.356 |
| Yes | 68 | 42 | 110 | ||
| Vascular tumor thrombus | |||||
| No | 39 | 17 | 56 | 1.235 | 0.266 |
| Yes | 57 | 37 | 94 | ||
| AFP (ng/mL) | |||||
| ≤8.1 | 89 | 51 | 140 | 0.077 | 0.782 |
| >8.1 | 6 | 2 | 8 | ||
| Unknown | 1 | 1 | 2 | ||
| CEA (ng/mL) | |||||
| ≤5 | 75 | 37 | 112 | 1.686 | 0.194 |
| >5 | 21 | 17 | 38 | ||
| CA19-9 (U/mL) | |||||
| ≤37 | 70 | 33 | 103 | 2.239 | 0.135 |
| >37 | 26 | 21 | 47 | ||
| CA72-4 (U/mL) | |||||
| ≤6.9 | 70 | 35 | 105 | 1.453 | 0.228 |
| >6.9 | 17 | 14 | 31 | ||
| Unknown | 8 | 6 | 14 | ||
| CA125 (U/mL) | |||||
| ≤35 | 91 | 46 | 137 | 2.907 | 0.088 |
| >35 | 5 | 8 | 13 | ||
| CA50 (U/mL) | |||||
| ≤25 | 52 | 24 | 76 | 6.495 | 0.011* |
| >25 | 6 | 11 | 17 | ||
| Unknown | 38 | 19 | 57 | ||
*, P<0.05. CerS5, ceramide synthase 5; GC, gastric cancer; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CA72-4, carbohydrate antigen 72-4; CA125, carbohydrate antigen 125; CA50, carbohydrate antigen 50.
In addition, we found that the high positive rate of carbohydrate antigen 50 in gastric cancer patients with high expression of CerS5 is 20.4% (11/54), and the high positive rate of CA50 in patients with low expression of CerS5 is only 6.3% (6/96) (Table 2, P=0.011). CA50, a non-specific broad-spectrum tumor marker, is mainly used for the auxiliary diagnosis of pancreatic cancer, CRC, and gastric cancer (22,23). These data showed that the expression level of CerS5 is positively correlated with the positive rate of CA50, suggesting that CerS5 is probably important for the auxiliary diagnosis of gastric cancer. Nevertheless, other indicators, such as age, gender, smoking history, drinking history, family history, TNM stage, Lauren classification, tumor size, tumor differentiation, CA125, and other common gastrointestinal tumor markers had no significant correlation with the expression level of CerS5 in gastric cancer.
The relevance of CerS5 to the prognosis of gastric cancer patients
To address the relevance of CerS5 levels to the prognosis of gastric cancer patients, we followed up 150 gastric cancer patients regularly to obtain survival data and used the Kaplan-Meier method to draw the survival curves. As shown in Figure 3A, the prognosis of gastric cancer patients with high expression levels of CerS5 was significantly worse than the low expression group. The 5-year survival rate of the low expression group was 52.8%, while the rate of the high expression group was only 32.8%. These results suggested that the expression level of CerS5 is negatively correlated with the prognosis of gastric cancer patients.
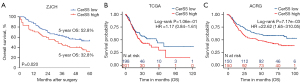
In order to verify the relevance of CerS5 to the prognosis of gastric cancer in a larger cohort, we collected 399 samples of gastric cancer patients from TCGA, and divided them into two different groups, the high expression group, and the low expression group, in terms of the levels of CerS5 (https://cdn.amegroups.cn/static/public/tcr-22-1348-2.xls). As shown in Figure 3B, CerS5 levels had no significant correlation with the prognosis of gastric cancer patients from TCGA.
Since TCGA is mainly based on the European population, we then collected the survival data of gastric cancer patients from ACRG, which is based on the Asian population, to further investigate the role of CerS5 in different populations. The samples of 300 gastric cancer patients and the corresponding survival data from ACRG (GSE66229, https://www.ncbi.nlm.nih.gov/geo) were analyzed by the Kaplan-Meier method. The results showed that the patients with high expression levels of CerS5 from the GSE66229 dataset had a worse prognosis than those with low expression levels of CerS5 (https://cdn.amegroups.cn/static/public/tcr-22-1348-3.xls, P=0.00717), suggesting that the expression level of CerS5 is significantly negatively correlated with the prognosis of gastric cancer patients in the Asian population (Figure 3C).
To address the correlation of CerS5 levels and clinicopathological features with the prognosis of gastric cancer patients, we used the univariate Cox regression method to analyze all the clinicopathological markers included in this study. As shown in Table 3, the expression level of CerS5 (P=0.023), family history of gastric cancer (P=0.008), Lauren classification (P=0.015), N stage (P<0.001), M stage (P<0.001), TNM stage (P=0.001) and vascular tumor thrombus (P=0.001), CEA (P=0.005), and CA125 (P<0.001) were correlated factors affecting the prognosis of gastric cancer patients. Subsequently, the factors with an index of P<0.1 by using univariate Cox regression analysis were subjected to multivariate Cox regression analysis. As shown in Table 4, CerS5 expression (P=0.046), Lauren classification (P=0.022), N stage (P=0.010), M stage (P=0.023), and CA125 (P=0.001) were independent factors, which are able to affect the prognosis of gastric cancer patients.
Table 3
| Parameters | Univariate Cox regression analysis | ||
|---|---|---|---|
| B value | P value | HR (95% CI) | |
| Gender | |||
| Female vs. male | 0.047 | 0.847 | 1.049 (0.647, 1.700) |
| Age (year) | |||
| ≤61 vs. >61 | 0.054 | 0.808 | 1.056 (0.682, 1.633) |
| CerS5 expression | |||
| Low vs. high | 0.513 | 0.023* | 1.671 (1.075, 2.596) |
| Family history (GC) | |||
| No vs. yes | 0.731 | 0.008* | 2.076 (1.212, 3.558) |
| Smoking history | |||
| No vs. yes | 0.140 | 0.544 | 1.150 (0.732, 1.807) |
| Drinking history | |||
| No vs. yes | 0.204 | 0.415 | 1.226 (0.751, 2.001) |
| Weight loss | |||
| No vs. yes | 0.271 | 0.231 | 1.312 (0.842, 2.044) |
| Tumor location | |||
| Proximal vs. distal vs. total | 0.151 | 0.468 | 1.163 (0.774, 1.747) |
| Lauren classification | |||
| Intestinal vs. diffuse vs. mixed | 0.354 | 0.015* | 1.425 (1.070, 1.897) |
| Tumor size (cm) | |||
| ≤5 vs. >5 | 0.481 | 0.063 | 1.618 (0.974, 2.688) |
| Grade of differentiation | |||
| Poor vs. moderate-poor vs. moderate | 0.116 | 0.448 | 0.890 (0.659, 1.203) |
| T stage | |||
| T1, T2 vs. T3, T4 | 1.640 | 0.103 | 5.155 (0.716, 37.105) |
| N stage | |||
| N0, N1 vs. N2, N3 | 1.120 | <0.001** | 3.066 (1.659, 5.664) |
| M stage | |||
| M0 vs. M1 | 1.290 | <0.001** | 3.633 (1.979, 6.668) |
| TNM stage | |||
| II vs. III vs. IV | 0.927 | 0.001* | 2.527 (1.496, 4.268) |
| Nerve invasion | |||
| No vs. yes | 0.511 | 0.068 | 1.667 (0.964, 2.884) |
| Vascular tumor thrombus | |||
| No vs. yes | 0.865 | 0.001** | 2.374 (1.431, 3.938) |
| AFP (ng/mL) | |||
| ≤8.1 vs. >8.1 | 0.160 | 0.707 | 1.173 (0.510, 2.697) |
| CEA (ng/mL) | |||
| ≤5 vs. >5 | 0.667 | 0.005* | 1.948 (1.223, 3.104) |
| CA19-9 (U/mL) | |||
| ≤37 vs. >37 | 0.443 | 0.054 | 1.558 (0.992, 2.446) |
| CA72-4 (U/mL) | |||
| ≤6.9 vs. >6.9 | 0.060 | 0.833 | 0.942 (0.542, 1.637) |
| CA125 (U/mL) | |||
| ≤35 vs. >35 | 1.403 | <0.001** | 4.068 (2.181, 7.586) |
| CA50 (U/mL) | |||
| ≤25 vs. >25 | 0.625 | 0.071 | 1.867 (0.949, 3.674) |
*, P<0.05; **, P<0.001. GC, gastric cancer; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CA72-4, carbohydrate antigen 72-4; CA125, carbohydrate antigen 125; CA50, carbohydrate antigen 50.
Table 4
| Parameters | Multivariate Cox regression analysis | ||
|---|---|---|---|
| B value | P value | HR (95% CI) | |
| CerS5 expression | |||
| Low vs. high | 0.667 | 0.046* | 1.948 (1.010, 3.754) |
| Family history (GC) | |||
| No vs. yes | 0.226 | 0.572 | 1.254 (0.572,2.748) |
| Lauren classification | |||
| Intestinal vs. diffuse vs. mixed | 0.573 | 0.022* | 1.774 (1.087, 2.893) |
| Tumor size (cm) | |||
| ≤5 vs. >5 | 0.239 | 0.524 | 1.270 (0.609, 2.650) |
| N stage | |||
| N0, N1 vs. N2, N3 | 1.433 | 0.010* | 4.190 (1.412, 12.435) |
| M stage | |||
| M0 vs. M1 | 1.936 | 0.023* | 6.932 (1.312, 36.633) |
| TNM stage | |||
| II vs. III vs. IV | 0.519 | 0.431 | 0.595 (0.164, 2.165) |
| Nerve invasion | |||
| No vs. yes | 0.497 | 0.200 | 0.609 (0.285, 1.300) |
| Vascular tumor thrombus | |||
| No vs. yes | 0.442 | 0.250 | 1.555 (0.733, 3.301) |
| CEA (ng/mL) | |||
| ≤5 vs. >5 | 0.879 | 0.057 | 2.407 (0.976, 5.939) |
| CA19-9 (U/mL) | |||
| ≤37 vs. >37 | 0.509 | 0.334 | 0.601 (0.214, 1.687) |
| CA125 (U/mL) | |||
| ≤35 vs. >35 | 1.786 | 0.001* | 5.964 (2.058, 17.279) |
| CA50 (U/mL) | |||
| ≤25 vs. >25 | 0.177 | 0.769 | 0.838 (0.257, 2.731) |
*, P<0.05. GC, gastric cancer; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125; CA50, carbohydrate antigen 50.
DiscussionOther Section
Gastric cancer is one of the most common human cancers worldwide, with low early diagnosis rates, poor outcomes of combined treatment, and low median OS rates (24). Most gastric cancer patients were diagnosed in the advanced stages, and around 1/3 of them had missed the opportunities for surgical treatment at the first diagnosis. Hence, the exploration of new strategies for diagnosis and treatment is the main way to achieve the improvement of therapeutic outcomes for gastric cancer patients. Emerging evidence has shown that gene mutations, epigenetic alterations, and signaling pathway disorders are closely related to the occurrence and development of gastric cancer (25-27). Several molecules or signaling pathways, which are of abnormal activation, have been found to be potential therapeutic targets for gastric cancer. Some of these newly discovered therapeutic targets were developed for disease screening, and some of them have achieved remarkable therapeutic effects in clinical trials (28,29). However, more effective strategies for early diagnosis and treatment for gastric cancer patients are remaining to be further explored.
CerS belong to a family composed of six mammalian enzymes, which was found in 2002 (30). Each enzyme has unique characteristics and generates ceramide with specific fatty acids, which are involved in a series of biological behaviors, such as cell proliferation, apoptosis, and autophagy (12,31-33). Many studies have shown that CerS play essential roles in the development of human cancers. Different subtypes of CerS have diverse functions in different kinds of tumors. Mojakgomo et al. found that Cers4 and CerS5 mRNA levels were overexpressed in endometrial cancer (EC) and CRC, while their expression levels decreased in response to apoptosis (34). Schiffmann et al. compared the concentrations of endogenous ceramide in biopsy tissues by using liquid chromatography tandem-mass spectrometry (LC-MS/MS), including 43 cases of malignant breast cancers and 21 cases of benign breast tumors, and found that the level of total ceramide averaged 12-fold and 4-fold higher than normal breast tissue samples, for malignant and benign tumors, respectively (35). Moreover, compared with estrogen receptor-negative breast cancer patients, the expression levels of CerS4 and CerS6 mRNA in estrogen receptor-positive patients were significantly upregulated, revealing the possibility that the levels of CerS were modulated in an estrogen-dependent manner in breast cancer (36). However, the mechanism of how altered ceramide levels affected the development of breast cancer is remaining to be uncovered.
In this study, CerS5 levels of 150 gastric cancer patients from our institute were determined by using immunohistochemistry. Compared with paracancerous tissues, CerS5 was upregulated in gastric cancer tissues. Moreover, the expression level of CerS5 in metastatic lymph nodes from 99 gastric cancer patients with lymph node metastasis was determined, and no significant differential expression level of CerS5 occurred between cancer tissues and metastatic lymph nodes (P=0.176). These data showed that CerS5 is upregulated in both cancer tissue and metastatic lymph node of gastric cancer patients. Furthermore, we collected RNA-seq data of gastric cancer patients from public databases, TCGA, and ACRG, and also found that CerS5 is highly expressed in gastric cancer patients from different populations. Therefore, CerS5 is universally overexpressed in cancer tissues of patients with gastric cancer.
Emerging evidence showed that the correlation of CerS with the prognosis of gastric cancer patients exists in different types of human cancers. Fitzgerald et al. found that the high expression of CerS5 in tumor tissues was significantly correlated with the poor prognosis of patients with CRC (17). Moro and colleagues carried out a clinical study on the metabolism of ceramide in breast cancer, and found that ceramide levels in breast cancer tissues were significantly higher than those in normal breast tissues and adjacent tissues. The prognosis of breast cancer patients with high levels of ceramide was significantly worse than those with low ceramide levels (37). On the other hand, CerS2, CerS3, CerS4, and CerS5 were overexpressed in non-small cell lung cancer (NSCLC) patients, and their levels were positively correlated with the prognosis of NSCLC patients (38). Hence, CerS probably has different effects on the prognosis for human cancers.
Herein, we analyzed the expression level of CerS5 in cancer tissues and the prognostic status of gastric cancer patients, and found that the high expression level of CerS5 was correlated with the poor prognosis of gastric cancer patients. Moreover, both univariate and multivariate Cox regression analyses confirmed that CerS5 is an independent risk factor for the poor prognosis of gastric cancer patients. Furthermore, we included larger cohorts to collect more gastric cancer patients from TCGA and ACRG databases in this study. Interestingly, CerS5 levels in TCGA, which mainly focused on the European and American populations, were upregulated in cancer tissues, however, were not significantly correlated with the prognosis of gastric cancer patients. The expression level of CerS5 in ACRG, which is mainly focused on the Asian population, was upregulated in cancer tissues and negatively correlated with the prognosis of patients with gastric cancer. Therefore, the relevance of CerS5 to the prognosis of gastric cancer patients was probably dependent on the populations.
In conclusion, our study showed that CerS5 is a differentially expressed gene in gastric cancer, and its expression level is significantly negatively correlated with the prognosis of gastric cancer patients in the Asian population. The expression of CerS5 is correlated with the distribution of gastric cancer and the positive rate of CA50, suggesting that CerS5 is a potential screening marker for the auxiliary diagnosis of gastric cancer. Moreover, the high expression levels of CerS5 in tumor and metastatic lymph nodes indicate that CerS5 is also a potential drug treatment target for gastric cancer, which is worth exploring in the future.
However, there are still many limitations to this study. Firstly, the patients included in this study are all from one center, which might affect the results due to the limited ability of diagnosis and treatment. Secondly, the number of cases included in the study is not large enough. Larger cohorts with cases from multiple centers need to be included. Thirdly, due to the shortage of the management of the clinical data previously, some clinical information of the patients was missing. The factors analyzed in this study cannot represent all aspects of the disease. Finally, this study only investigated the relevance of CerS5 levels to the prognosis based on the retrospective data of gastric cancer patients. The mechanism of how CerS5 affects the development of gastric cancer is remaining to be addressed in the future.
ConclusionsOther Section
CerS5 is universally overexpressed in cancer tissues, and its expression level is negatively correlated with the prognosis of gastric cancer patients in the Asian population.
AcknowledgmentsOther Section
Funding: This work was supported by the
FootnoteOther Section
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1348/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1348/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1348/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee board of the Zhejiang Cancer Hospital (IRB-2021-431) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555-67. [Crossref] [PubMed]
- Solsky I, In H. Surgical Treatment for Gastric Cancer. Gastrointest Endosc Clin N Am 2021;31:581-605. [Crossref] [PubMed]
- Yoneda A, Kuroki T, Eguchi S. Immunotherapeutic advances in gastric cancer. Surg Today 2021;51:1727-35. [Crossref] [PubMed]
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 2020;396:635-48. [Crossref] [PubMed]
- Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol 2020;18:534-42. [Crossref] [PubMed]
- Huang RJ, Hwang JH. Improving the Early Diagnosis of Gastric Cancer. Gastrointest Endosc Clin N Am 2021;31:503-17. [Crossref] [PubMed]
- Stiban J, Tidhar R, Futerman AH. Ceramide synthases: roles in cell physiology and signaling. Adv Exp Med Biol 2010;688:60-71. [Crossref] [PubMed]
- Castro BM, Prieto M, Silva LC. Ceramide: a simple sphingolipid with unique biophysical properties. Prog Lipid Res 2014;54:53-67. [Crossref] [PubMed]
- Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J 2012;441:789-802. [Crossref] [PubMed]
- Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 2018;18:33-50. [Crossref] [PubMed]
- Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 2013;13:51-65. [Crossref] [PubMed]
- Fan SH, Wang YY, Lu J, et al. CERS2 suppresses tumor cell invasion and is associated with decreased V-ATPase and MMP-2/MMP-9 activities in breast cancer. J Cell Biochem 2015;116:502-13. [Crossref] [PubMed]
- Suzuki M, Cao K, Kato S, et al. Targeting ceramide synthase 6-dependent metastasis-prone phenotype in lung cancer cells. J Clin Invest 2019;129:5050. [Crossref] [PubMed]
- Brachtendorf S, El-Hindi K, Grösch S. Ceramide synthases in cancer therapy and chemoresistance. Prog Lipid Res 2019;74:160-85. [Crossref] [PubMed]
- Chen L, Chen H, Li Y, et al. Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol Rep 2015;34:447-54. [Crossref] [PubMed]
- Fitzgerald S, Sheehan KM, Espina V, et al. High CerS5 expression levels associate with reduced patient survival and transition from apoptotic to autophagy signalling pathways in colorectal cancer. J Pathol Clin Res 2014;1:54-65. [Crossref] [PubMed]
- Jiang Z, Li F, Wan Y, et al. LASS5 Interacts with SDHB and Synergistically Represses p53 and p21 Activity. Curr Mol Med 2016;16:582-90. [Crossref] [PubMed]
- Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life 2010;62:347-56. [PubMed]
- Brachtendorf S, Wanger RA, Birod K, et al. Chemosensitivity of human colon cancer cells is influenced by a p53-dependent enhancement of ceramide synthase 5 and induction of autophagy. Biochim Biophys Acta Mol Cell Biol Lipids 2018;1863:1214-27. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Holmgren J, Lindholm L, Persson B, et al. Detection by monoclonal antibody of carbohydrate antigen CA 50 in serum of patients with carcinoma. Br Med J (Clin Res Ed) 1984;288:1479-82. [Crossref] [PubMed]
- Shan M, Tian Q, Zhang L. Serum CA50 levels in patients with cancers and other diseases. Prog Mol Biol Transl Sci 2019;162:187-98. [Crossref] [PubMed]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264-79. [Crossref] [PubMed]
- Jin G, Lv J, Yang M, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol 2020;21:1378-86. [Crossref] [PubMed]
- Grady WM, Yu M, Markowitz SD. Epigenetic Alterations in the Gastrointestinal Tract: Current and Emerging Use for Biomarkers of Cancer. Gastroenterology 2021;160:690-709. [Crossref] [PubMed]
- Chen B, Chan WN, Mui CW, et al. STK3 promotes gastric carcinogenesis by activating Ras-MAPK mediated cell cycle progression and serves as an independent prognostic biomarker. Mol Cancer 2021;20:147. [Crossref] [PubMed]
- Jiang L, Gong X, Liao W, et al. Molecular targeted treatment and drug delivery system for gastric cancer. J Cancer Res Clin Oncol 2021;147:973-86. [Crossref] [PubMed]
- Li Y, Qin C. MiR-1179 inhibits the proliferation of gastric cancer cells by targeting HMGB1. Hum Cell 2019;32:352-9. [Crossref] [PubMed]
- Venkataraman K, Riebeling C, Bodennec J, et al. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J Biol Chem 2002;277:35642-9. [Crossref] [PubMed]
- Bose R, Verheij M, Haimovitz-Friedman A, et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell 1995;82:405-14. [Crossref] [PubMed]
- Li G, Liu D, Kimchi ET, et al. Nanoliposome C6-Ceramide Increases the Anti-tumor Immune Response and Slows Growth of Liver Tumors in Mice. Gastroenterology 2018;154:1024-1036.e9. [Crossref] [PubMed]
- Muñoz-Guardiola P, Casas J, Megías-Roda E, et al. The anti-cancer drug ABTL0812 induces ER stress-mediated cytotoxic autophagy by increasing dihydroceramide levels in cancer cells. Autophagy 2021;17:1349-66. [Crossref] [PubMed]
- Mojakgomo R, Mbita Z, Dlamini Z. Linking the ceramide synthases (CerSs) 4 and 5 with apoptosis, endometrial and colon cancers. Exp Mol Pathol 2015;98:585-92. [Crossref] [PubMed]
- Schiffmann S, Sandner J, Birod K, et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis 2009;30:745-52. [Crossref] [PubMed]
- Wegner MS, Wanger RA, Oertel S, et al. Ceramide synthases CerS4 and CerS5 are upregulated by 17β-estradiol and GPER1 via AP-1 in human breast cancer cells. Biochem Pharmacol 2014;92:577-89. [Crossref] [PubMed]
- Moro K, Kawaguchi T, Tsuchida J, et al. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 2018;9:19874-90. [Crossref] [PubMed]
- Qian H, Deng J, Lu C, et al. Ceramide synthases: insights into the expression and prognosis of lung cancer. Exp Lung Res 2021;47:37-53. [Crossref] [PubMed]


