
Anlotinib combined with temozolomide for the treatment of patients with diffuse midline glioma: a case report and literature review
Introduction
Diffuse midline glioma incorporating a histone H3-K27M mutation is recognized as a new diagnostic entity in 2016 edition of the World Health Organization (WHO) Classification of Tumors of the Central Nervous System (1). Although these gliomas primarily occur in children, they can also occur in adults. In children, H3-K27M mutant gliomas are frequently present within the pons, although in adults, they appear more commonly within the spinal cord and the thalamus. Diffuse midline gliomas are incurable and aggressive brain tumors, characterized by high levels of intrinsic and acquired resistance to therapy, as well as therapy failure due to an intact blood-brain barrier, leading to a dismal median survival of about 11 months (2).
Anlotinib is a multitarget, novel tyrosine kinase inhibitor that interacts with angiogenesis-related kinases such as fibroblast growth factor receptors (FGFRs) 1/2/3, vascular endothelial growth factor receptor (VEGFR) 1/2/3, and other tumor-associated kinases such as c-Kit and Ret (3). Anlotinib has been used for the treatment of sarcoma, non-small cell lung cancer and metastatic renal cell carcinoma, with satisfying outcomes (4). However, anlotinib has not been reported for the treatment of patients with diffuse midline glioma. Here we report a case of a male adult patient with diffuse midline glioma with histone H3-K27M mutation treated with a combination of anlotinib and temozolomide (TMZ). We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1073/rc).
Case presentation
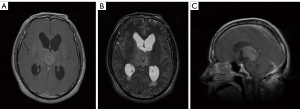
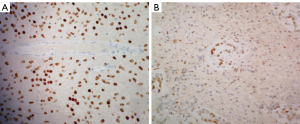
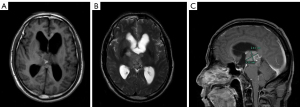
A 51-year-old man was first admitted to our hospital complaining of headache and lower limb weakness for the past 2 months. Magnetic resonance imaging (MRI) revealed an oval abnormal lesion in the pineal region, about 23 mm × 28 mm mm in size. The mass was hypointensive on T1-weighted imaging, hyperintensive on T2, and strongly enhanced by gadolinium-based contrast MRI (Figure 1). The patient underwent a stereotactic biopsy as well as Ommaya reservoir placement. Pathology reported diffuse midline glioma (WHO Grade IV), H3-K27M mutation, and immunohistochemistry showed GFAP (+), olig2 (−/+), p53 (−/+), ATRX (+), IDH1 (−), Syn (+), Neun (+), Ki67 (6%+), H3K27M (+), and H3K27me3 (+) (Figure 2). Further results of gene tests indicated MGMT promoter methylation (−), 1p/19q co-deletion (−), IDH1/2 mutation (−), TERT C228T mutation (+), TERT C250T mutation (−), and BRAF V600E mutation (−). In addition, copy-number variations presented gene amplification in KDR, KIT, MDM4 and PDGFRA genes.
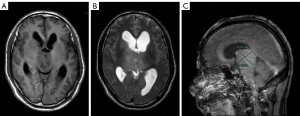
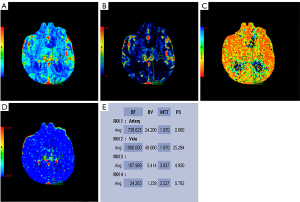
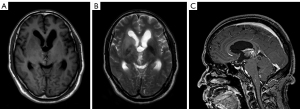
After surgery, the patient underwent chemoradiation treatment (Stupp Protocol), and then adjuvant TMZ (5 days out of a 28-day cycle); 7 months later, the patient had symptoms of headache, nausea, vomiting, weakness in the limbs, memory impairment and uracratia. The MRI scan showed that the tumor had enlarged with severe peritumor edema and hydrocephalus (Figure 3). Furthermore, CT perfusion examination revealed that tumor activity existed in the mass (Figure 4). In addition to extraventricular drainage via Ommaya reservoir, therapy with bevacizumab 5 mg/kg (once every 3 weeks), was carried out. His condition improved with Karnofsky performance score (KPS) 90 after 3 cycles of therapy. MRI demonstrated that the volume of tumor had decreased and the ventricles had shrunk (Figure 5). Considering that it was inconvenient for patients to inject bevacizumab, we recommended adjusting the treatment plan to TMZ (200 mg/m2, qd d1–5, 28 days a cycle) plus oral administration of anlotinib (10 mg/m2, qd d1–14, 21 days a cycle). The cerebrospinal fluid next generation sequencing (NGS) test revealed CDKN2B missense, H3F3A missense, KDR missense and NF1 frameshift mutations, as well as PPM1D nonsense mutation, IDH1/2 nonmutation, and RELA nonmutation. The patient had a good tolerance to anlotinib except for high blood pressure, which was controlled by an anti-hypertensive drug. No more than grade 3 side effects were exhibited. Re-examination of MRIs every 3 months showed that the volume of the tumor mass continuously decreased. The 9 months follow-up MRI examination concluded that the mass was stable (Figure 6); 11 months after anlotinib combined with TMZ treatment, the patient died of pneumonia caused by aspiration.
All procedures performed in this study were in accordance with the ethical standards of the Clinical Research Ethics Committee of The First Affiliated Hospital College of Medicine, Zhejiang University (approval No. IIT20200011c) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Diffuse midline glioma with histone H3-K27M mutation is classified as WHO Grade IV, which is located mainly in the brain stem (previously known as brain stem glioma), the thalamus, and the spinal cord (previously known as diffuse intrinsic pontine glioma) (1). Mutation of the H3F3A gene or less commonly the HIST1H3B gene, which separately encodes histone H3 variants H3.3 and H3.1, contributes to histone H3-K27M mutation in diffuse midline glioma. A recurrent lysine to methionine substitution at codon 27 (K27M) is present in these histone variants mainly in diffuse intrinsic pontine gliomas and thalamic glioblastomas (GBM) according to early reports (5,6). A recent study (7) revealed that the H3-K27M mutation appears in the majority of midline structures (thalamus, pons and spinal cord) in both pediatric and adult patients.
As indicated previously, treatment of patients with diffuse midline glioma has not yet produced satisfactory outcomes. Total resection of tumor is difficult to achieve, conventional cytotoxicity drugs have limited effects on treatment due to the blood brain barrier, and 2-year survival is <10%, even though these patients received regular chemotherapy and radiotherapy (8). Under such circumstances, multiple regimens involving combination therapy (9), targeted therapy, and immunotherapy (10) have been trialed, with promising results.
Anti-VEGF drugs have been widely used to treat various malignant tumors, including high grade glioma. Glioma is one of the most vascularized tumors and its cells can produce VEGF, elevated concentrations of which usually induces a worse prognosis in patients diagnosed with cancer. Bevacizumab, a monoclonal antibody against VEGF, has become the standard treatment of choice for patients with high-grade glioma in the National Comprehensive Cancer Network (NCCN) guidelines (11). Previous research revealed that bevacizumab combined with chemotherapy can significantly improve progression-free survival (PFS) in comparison with bevacizumab alone, but does not prolong overall survival (OS) (12). Liu et al. (13) reported that endothelial-mesenchymal transformation induces GBM resistance to antiangiogenic therapy by downregulating VEGFR-2 expression in tumor-associated endothelial cells. This latter result could explain why the efficacy of anti-VEGF was poor in this case. Moreover, bevacizumab was very effective in improving radiation injury. Deibert et al. (14) retrospectively evaluated the clinical benefit and imaging response of bevacizumab when used to treat refractory adverse radiation effects (ARE) after stereotactic radiosurgery. Their study of 29 patients with brain tumors or vascular malformations concluded that bevacizumab could reduce both the symptoms and reactive imaging changes in patients with ARE. After stereotactic radiosurgery (SRS), refractory ARE unresponsive to initial corticosteroids or other agents may benefit from bevacizumab therapy, which was consistent with our findings.
In addition to bevacizumab, several other anti-angiogenic drugs have been reported for the treatment of patients with gliomas. Duerinck et al. (15) demonstrated that axitinib improved the response rates and PFS in patients with recurrent GBM compared to historical controls. This noncomparative randomized phase 2 clinical trial investigated axitinib monotherapy versus axitinib plus lomustine therapy in patients with recurrent GBM, and reported no clinical benefit from this combination of drugs. A randomized, multicenter, open-label phase 2 trial (16) in Italy showed an encouraging OS benefit of regorafenib in recurrent GBM. OS was significantly improved (P=0.001) in the regorafenib group (7.4 months) compared to the lomustine group (5.6 months). A phase 2 study reported that cediranib, an oral anti-VEGF receptor tyrosine kinase inhibitor, was administered to 31 patients with recurrent GBM. Cediranib monotherapy for recurrent GBM was associated with encouraging proportions of radiographic responses (56.7%), 6-month PFS and a steroid-sparing effect with manageable toxicity (17).
Anlotinib is a newly developed, orally active, small-molecule RTK inhibitor that targets VEGFR1, VEGFR2/KDR, VEGFR3, c-Kit, PDGFR-α and FGFRs. Anlotinib can inhibit both tumor angiogenesis and tumor cell proliferation (3) and can inhibit more targets and has a better antiangiogenic activity than other drugs. The therapeutic efficacy of anlotinib has been proved in advanced medullary thyroid cancer, metastatic renal cell carcinoma, advanced soft tissue sarcoma and advanced non-small-cell lung cancer (18-21). Only mild side effects were discovered in these studies. These adverse events mainly include hypertension, elevated thyroid-stimulating hormone, hand and foot syndrome, elevated thyroglobulin, elevated total cholesterol, etc. (18-21). However, in other investigations, researchers tried to obtain superior outcomes for the use of anlotinib in the treatment of high-grade glioma. Lv et al. (22) reported a case of a patient with recurrent GBM treated with anlotinib. After anlotinib therapy, the visual acuity of the patient was immensely increased and her OS reached 110 days, though the tumor progressed again after 2 months.
Our case used anlotinib combined with TMZ for 11 months, exhibited good tolerance to the therapy and suffered no severe side effects. The survival period of the patient was 20 months. It is worth mentioning that the patient cerebrospinal fluid (CSF) NGS revealed gene amplification in KDR, KIT and PDGFRA genes, which thereby explained the excellent efficacy of anlotinib, Obviously, abnormal expression of KDR, KIT, and PDGFRA is involved in the process of tumorigenesis, and these molecules are all targeted by anlotinib. Except for the advantage of its multitarget activity, anlotinib can alleviate pain by replacing the need for injection with oral intake, which saves both time and money spent on patient travel from home to hospital, which is a great convenience for patients.
Therapy combining anlotinib with TMZ provides a new option for the treatment of patients with diffuse midline glioma. However, the efficacy and side effects of this drug combination for high grade glioma still requires large-scale clinical trials to confirm this assertion.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1073/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1073/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Clinical Research Ethics Committee of The First Affiliated Hospital College of Medicine, Zhejiang University (approval No. IIT20200011c) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Jones C, Karajannis MA, Jones DTW, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol 2017;19:153-61. [PubMed]
- Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 2018;109:1207-19. [Crossref] [PubMed]
- Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. [Crossref] [PubMed]
- Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012;482:226-31. [Crossref] [PubMed]
- Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 2012;44:251-3. [Crossref] [PubMed]
- Aihara K, Mukasa A, Gotoh K, et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol 2014;16:140-6. [Crossref] [PubMed]
- Buczkowicz P, Bartels U, Bouffet E, et al. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 2014;128:573-81. [Crossref] [PubMed]
- Frazier JL, Lee J, Thomale UW, et al. Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr 2009;3:259-69. [Crossref] [PubMed]
- Mount CW, Majzner RG, Sundaresh S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat Med 2018;24:572-9. [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733-40. [Crossref] [PubMed]
- Liu T, Ma W, Xu H, et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat Commun 2018;9:3439. [Crossref] [PubMed]
- Deibert CP, Ahluwalia MS, Sheehan JP, et al. Bevacizumab for refractory adverse radiation effects after stereotactic radiosurgery. J Neurooncol 2013;115:217-23. [Crossref] [PubMed]
- Duerinck J, Du Four S, Bouttens F, et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J Neurooncol 2018;136:115-25. [Crossref] [PubMed]
- Lombardi G, De Salvo GL, Brandes AA, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 2019;20:110-9. [Crossref] [PubMed]
- Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 2010;28:2817-23. [Crossref] [PubMed]
- Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018;118:654-61. [Crossref] [PubMed]
- Chi Y, Fang Z, Hong X, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res 2018;24:5233-8. [Crossref] [PubMed]
- Zhou AP, Bai Y, Song Y, et al. Anlotinib Versus Sunitinib as First-Line Treatment for Metastatic Renal Cell Carcinoma: A Randomized Phase II Clinical Trial. Oncologist 2019;24:e702-8. [Crossref] [PubMed]
- Sun Y, Du F, Gao M, et al. Anlotinib for the Treatment of Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer. Thyroid 2018;28:1455-61. [Crossref] [PubMed]
- Lv Y, Zhang J, Liu F, et al. Targeted therapy with anlotinib for patient with recurrent glioblastoma: A case report and literature review. Medicine (Baltimore) 2019;98:e15749. [Crossref] [PubMed]


