
Is it justified to assess the resectability of pancreatic cancer combined with biological and conditional factors?
Introduction
As one of the most rapidly-growing and lethal cancers, pancreatic ductal adenocarcinoma (PDAC) is expected to become the second leading cause of deaths in developed countries within the next decade (1). To date, curative resection, combined with systemic treatment, offers the only chance for prolonged survival for a minority of patients. In fact, less than 20% of PDAC patients are diagnosed with a resectable disease (2). Among patients with PDAC who are candidates for upfront surgery, more than 80% experience tumor recurrence after curative resection, resulting in 5-year survival rates of 20–30%, even at high volume centers (3,4). The dismal outcomes of PDAC have been largely attributed to the presence of occult micro-metastases prior to surgery, as well as a lack of systemic treatment (4-7). This concerning situation has triggered a lot of discussion on a broader resectability criteria for pancreatic cancer.
In order to improve the patient’s selection for upfront surgery, the resectability criteria of PDAC have been developed by different guidelines (8). Additionally, tremendous advances in imaging techniques have made the resectability assessment of PDAC more intensive. In 2001, the concept of “marginal resectable” was initially proposed by Mehta et al. (9) for a tumor that has a high risk of margin positivity after an upfront pancreas resection. In 2006, the concept of “borderline resectable (BR)” was adopted by the National Comprehensive Cancer Network (NCCN) guidelines for the first time, and neoadjuvant therapy was recommended for tumors that were classified as BR. In the updated NCCN guideline for PDAC, the resectability status was classified as resectable, BR, or locally advanced (LA), based primarily on the anatomic relationship between tumor and major vessels, which has been widely used. Nevertheless, in addition to the risk of positive margins, the biological behavior of the tumor, as well as the patient’s performance, must be considered during the decision-making process of surgery. These biological behaviors are closely related to the preoperative risk of occult tumor metastasis, and the postoperative risk of severe complications and death. Biological and conditional criteria for BR pancreatic cancer were initially presented in 2008 by Katz et al. (10), but were not adopted by clinical practice guidelines. In 2016, the 20th International Association of Pancreatology (IAP) meeting was held in Sendai, Japan and a consensus was reached on a broader criterium for BR-PDAC (8), defined from anatomical (A), biological (B), and conditional (C) dimensions (i.e., IAP criteria). Hence, the relationship between tumor and major vessels, preoperative serum CA19-9 levels, lymph node status indicated by preoperative biopsy or positron emission tomography (PET)-computed tomography (CT) and the patient’s preoperative Eastern Cooperative Oncology Group (ECOG) score were included in the resectability assessment (8). The IAP criteria indicate a new standard for expanding the resectability criteria of PDAC. Most relevant studies have endorsed the function of biological and individual factors in the preoperative resectability assessment of PDAC (11-13). However, the criteria remain controversial with regard to the selection of inclusion factors, the cut-off value of CA19-9, and assessment methods of preoperative lymph node metastasis and patient performance.
Reasonable preoperative assessment of resectability is important for accurate clinical decision-making and improving the prognosis of PDAC patients. Thus, a single-center retrospective study was carried out with the objective to assess the biological and conditional resectability criteria of BR-PDAC, as proposed by IAP, as well as to investigate the function of biological and conditional factors in evaluating the resectability of PDAC. The hypothesis of this retrospective study was that even if the patient was diagnosed as anatomically resectable PDAC according to NCCN criteria and underwent radical resection, the prognosis was still poor due to unfavorable biological and conditional factors. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1258/rc).
Methods
Patient selection
Patients with a histological diagnosis of PDAC who underwent upfront abdominal/laparoscopic pancreaticoduodenectomy (PD/LPD) or distal pancreatectomy (DP/LDP) at the Second Affiliated Hospital of Zhejiang University between January 2013 to June 2019 were reviewed. Among the cohort, patients with anatomically resectable tumors were identified and then enrolled based on the NCCN guideline Version 1.2020. The anatomically resectable PDAC was defined as either no tumor contact with a superior mesenteric artery (SMA)/coeliac axis (CA)/common hepatic artery (CHA) and portal vein (PV)/superior mesenteric vein (SMV), or with a less than 180° contact with PV/SMV, and without vein contour irregularity on CT imaging.
Exclusion criteria included diagnosis of any other malignancies, having a BR and unresectable tumor (NCCN-criteria), having received any neoadjuvant therapy, having a grossly positive resection margin (R2), death within 90 days after surgery or incomplete medical records. Patients who completed follow-up at other institutions were also excluded.
Data collection and treatment
All data were collected from the electronic medical record system which included patient characteristics (i.e., sex, age at time of surgery and operation type). ECOG score and body mass index (BMI) were assessed when the patients were admitted for surgery. Changes in body weight within 3 months before surgery and onset symptoms, including abdominal pain and jaundice, were also utilized to evaluate the performance status of enrolled cases. Preoperative serum albumin levels (normal range: ≥35 g/L) and C-reactive protein (CRP) levels (normal range: <10 mg/L) were recorded, and utilized to calculate the modified Glasgow prognostic score (mGPS). The scoring criteria were as follows. A score of 0 indicated that the patient had normal CRP levels. A score of 1 indicates that the patient had elevated CRP without hypoproteinemia. A score of 2 indicates that the patient had elevated CRP with hypoproteinemia. The mGPS was utilized for preoperative assessment of systemic inflammatory response, and nutritional status, among patients with pancreatic cancer (14). Preoperative tumor marker values, such as serum CA19-9 (normal range: <37 U/mL), were obtained within 1 month prior to curative resection. The preoperative radiological evaluation consisted of chest CT, abdominal contrast-enhanced CT, magnetic resonance imaging (MRI) and/or PET-CT.
Next, patients were retrospectively grouped [IAP-resectable (IAP-R) and IAP-BR] according to a consensus statement by IAP, based on the definition and criteria of BR PDAC (8).
Patients with preoperative CA19-9 serum levels that exceeded 500 U/mL and/or with an imaging diagnosis of regional lymph nodes metastasis (LNM) were assigned to the BR group as biological borderline resectable (BR-B). Preoperative PET-CT or biopsy of lymph nodes was not routinely carried out in all patients in this retrospective cohort. Thus, regional LNM was determined through preoperative enhanced CT imaging or PET-CT if there was an enlargement of more than 10 mm in the shorter diameter (8,13), homogeneous contrast enhancement or a higher fludeoxyglucose (FDG) uptake. Regarding the conditional dimension, patients whose ECOG score >1 were allocated to the BR group as conditional borderline resectable (BR-C), according to IAP-criteria.
Therapeutic decisions and surgical plans were carried out by a multidisciplinary team (MDT), including an attending surgeon, radiologist and pathologist. The procedures were conducted by experienced hepato-biliary-pancreatic surgeons that were within the same group. The surgical specimens were evaluated after a curative resection, tumor size, lymph node status, differentiation grade, and presence of perineural invasion, which was further described by an experienced pathologist. Lymph node ratio (LNR) refers to the number of positive nodes that are divided by the number of lymph nodes harvested. Microscopic margin involvement (R1) was defined as tumor cells that were present at the resection margin (1 mm clearance). TNM classification was determined through 8th International Union Against Cancer/American Joint Committee (UICC/AJCC) staging system for pancreatic cancer. Adjuvant chemotherapy refers to a patient who underwent at least one cycle of systemic chemotherapy (oncologists’ choice) after undergoing a radical surgery.
Follow-up
Contrast-enhanced CT or MRI of the full abdomen and chest was observed at least every three to 6 months for the first 2 years after surgery, and at least every 6 months thereafter. Chest CT scan or systemic fluorine-18 fluorodeoxyglucose PET was carried out when tumor recurrence or metastasis was suspected, or if there was a clinical indication. “Local failure” was defined as developing recurrence in the operative fields. “Distant recurrence” was defined as recurrence within the liver, lung and other distant sites. Early recurrence was defined as relapse within 12 months after undergoing a curative resection.
The primary endpoint of this study was to evaluate the difference in recurrence-free survival (RFS) between the IAP-R and IAP-BR groups. RFS was calculated from the date of surgery to the date of recurrence, or at last follow-up, if there was no recurrence. Overall survival (OS) was defined as the date of surgery to the date of death or last follow-up. Patients with less than 12 months of follow-up were excluded if neither recurrence nor death had occurred.
Statistical analysis
Categorical variables were presented as numbers and percentages. Fisher’s exact test or Pearson Chi-square test were performed, when appropriate. Continuous data are presented as median (range). Independent t-tests or Mann-Whitney U-tests were performed when appropriate.
Median RFS and OS were estimated with a Kaplan-Meier curve and compared with log-rank test. To ensure homogeneity and accuracy of OS analysis, patients with less than 12 months follow-up were further excluded from the OS analysis set, in which recurrence had observed but not death. In order to identify independent predictors of RFS and OS of anatomically resectable PDAC, an analysis using the Cox proportional-hazard model was carried out. P value <0.10 in univariate analysis was included in multivariate regression analysis as a covariate.
Patients with missing data were excluded prior to any analyses. P value <0.05 was statistically significant. The x-tile software (version 3.6.1) was utilized to determine the optimal cut-off value of maximum tumor diameter. MedCalc (MedCalc 15.2.2 version, MedCalc Inc., Mariakerke, Belgium) was utilized for statistical analysis and plotting.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University (No. 2020-506) and informed consent exemption had been obtained.
Results
Patient cohort
Between January 2013 to June 2019, 215 patients underwent upfront pancreatic resection and were histologically diagnosed with PDAC. Among them, 97 (45.1%) patients who were compatible with the inclusion criteria were enrolled into this cohort.
Among patients who were excluded, 42 (19.5%) patients were classified as being BR or LA tumor and 8 (3.7%) patients were classified as having an unresectable tumor, according to NCCN-criteria. Additionally, 66 (30.7%) patients whose radiological follow-up was not performed at our institution, or without any evidence of recurrence or death, were further excluded due to less than 12 months follow-up. Importantly, 2 (1.0%) patients who were classified as having a resectable tumor, and underwent total pancreatectomy, were not entered into the study.
Patients were grouped according to IAP criteria. Therefore, there were 38 (39.2%) in the resectable group (IAP-R), 59 (60.8%) in the borderline-resectable group (IAP-BR), including 56 (57.7%) in the BR-B group (IAP BR-B), and 3 (3.1%) in the BR-C group (IAP BR-C). Another five cases (5.2%) were diagnosed with both BR-B and BR-C (IAP BR-BC). The median age of all enrolled patients was 65 years old (range, 32–79 years old), and had a male-to-female ratio of 1.8:1.
For the entire cohort, the median RFS was 11.7 months (95% CI: 9.5–13.9 months). Local recurrence occurred in 22 (22.7%) patients, distant metastases in 43 patients (44.3%), and local recurrence combined with distant metastases in 8 (8.2%) patients. Furthermore, early recurrence was observed in 53 patients, which accounted for 69.7% of recurrences. A total of 79 patients were included in the OS analysis set, and the median OS was 24.0 months (95% CI: 18.2–29.7 months). The 1-, 3-, and 5-year survival rates were 84.8%, 55.6%, and 25.9%, respectively. Demographics, clinicopathological characteristics, and outcomes of the two groups are summarized in Table 1.
Table 1
| Characteristics at baseline | IAP-R (n=38) | IAP-BR (n=59) | P value |
|---|---|---|---|
| Sex, female | 14 (36.8) | 21 (35.6) | 0.90 |
| Age (years) | 63.5 [32–79] | 65 [45–79] | 0.35 |
| Clinical condition on diagnosis | |||
| ECOG score, 0–1 | 38 | 51 (86.4) | 0.02 |
| BMI (kg/m2) | 22.5 [17.5–34.2] | 22.1 [14.6–34.6] | 0.84 |
| mGPS >0 | 5 (13.2) | 18 (30.5) | 0.057 |
| Weight loss | 11 (28.9) | 27 (45.8) | 0.03 |
| Abdominal pain | 21 (55.3) | 26 (44.1) | 0.28 |
| Jaundice | 14 (36.8) | 13 (22.0) | 0.11 |
| Tumor anatomy and biology on diagnosis | |||
| Location, head of pancreas | 26 (68.4) | 44 (74.6) | 0.51 |
| Tumor size (cm) | 2.1 [1.0–5.5] | 3.0 [1.0–7.0] | <0.01 |
| CA19-9 (U/mL) | 63.3 [6.0–439.0] | 751.0 [2.0–12,000.0] | <0.01 |
| LNM (+) | 0 | 25 | <0.01 |
| PV/SMV abutment | 4 (10.5) | 10 (16.9) | 0.38 |
| Post-operative clinical characteristics | |||
| Type of surgery | 0.51 | ||
| PD/LPD | 24/1 (65.8) | 39/5 (74.6) | |
| DP/LDP | 12/1 (34.2) | 13/2 (25.4) | |
| pT stage, AJCC 8th | 0.005 | ||
| 1 | 17 (44.7) | 9 (15.2) | |
| 2 | 17 (44.7) | 37 (62.7) | |
| 3 | 4 (4.1) | 13 (22.0) | |
| pN Stage AJCC 8th | 0.08 | ||
| N1 | 13 (34.2) | 18 (30.5) | |
| N2 | 2 (5.3) | 13 (22.0) | <0.05 |
| AJCC 8th stage | 0.15 | ||
| I | 19 (50.0) | 24 (40.7) | |
| II | 17 (44.7) | 22 (37.3) | |
| III | 2 (5.3) | 12 (20.3) | |
| Adjuvant chemotherapy | 26 (68.4) | 44 (74.6) | 0.50 |
| Pathologic findings | |||
| LNR, >0.1 | 3 (7.9) | 19 (32.2) | <0.01 |
| Resection margin, R1 | 6 (15.8) | 5 (8.5) | 0.33 |
| Tumor differentiation, poor | 14 (36.8) | 22 (37.3) | 0.91 |
| Perineural invasion (+) | 4 (10.5) | 11 (18.6) | 0.85 |
| Recurrence pattern | 0.84 | ||
| Local | 8 (33.3) | 14 (28.6) | |
| Distant | 13 (54.2) | 30 (61.2) | |
| Local and distant | 3 (12.5) | 5 (10.2) | |
| Early recurrence (<12 months) | 16 (42.1) | 37 (62.7) | 0.047 |
| RFS, months | 18.3 [4.2–63.7] | 9.2 [1.4–55.3] | 0.004 |
| OS, months | 30.8 [9.0–72.3] | 19.4 [6.0–65.0] | 0.038 |
Data are expressed as median [range], number (%) or number. IAP, International Association of Pancreatology; R, resectable; BR, borderline resectable; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; mGPS, modified Glasgow prognostic score; LNM, lymph node metastasis; PV, portal vein; SMV, superior mesenteric vein; PD/LPD, pancreaticoduodenectomy/laparoscopic pancreaticoduodenectomy; DP/LDP, distal pancreatectomy/laparoscopic distal pancreatectomy; AJCC, American Joint Committee on Cancer; LNR, lymph node ratio; RFS, recurrence-free survival; OS, overall survival.
Preoperative anatomical, biological and conditional factors
With regards to tumor anatomical factors, preoperative imaging results revealed the presence of 69 (71.1%) tumors in the head of the pancreas, with a median maximum tumor diameter of 3.0 cm (range, 1.0–7.0 cm). Furthermore, tumor contact with the PV and/or SMV was observed in a total of 14 (14.4%) cases. Compared to the IAP-R group, the IAP-BR group had relatively larger tumors (3.0 vs. 2.1 cm, P<0.01). It should be noted that the anatomical resectability criteria of NCCN differ from the IAP criteria (Table 2), but all the included patients met the IAP anatomical resectable criteria as well as the NCCN by retrospective assessment: although preoperative CT imaging in 14 (14.4%) subjects suggested tumor contact with PV/SMV, but no PV/SMV contact 180° or greater and no venous vein contour irregularity.
Table 2
| Guidelines | Anatomical | Biological | Conditional |
|---|---|---|---|
| IAP 2016, (8) | BR-PV (SMV/PV involvement alone) | Clinical findings suspicious but nor proven distant metastasis, including CA19-9 level more than 500 units/mL, or regional lymph nodes metastasis diagnosed by biopsy or PET-CT | Performance status of 2 or more |
| ❖ SMV/PV: tumor contact 180° or greater or bilateral narrowing/occlusion, not exceeding the inferior border of the duodenum | |||
| ❖ SMA, CA, CHA: no tumor contact/invasion | |||
| BR-A (arterial involvement) | |||
| ❖ SMA, CA: tumor contact of less than 180° without showing deformity/stenosis | |||
| ❖ CHA: tumor contact without showing tumor contact of the PHA and/or CA | |||
| NCCN Guidelines Version 1. 2022, (15) | Arterial: | None | None |
| ❖ Pancreatic head/uncinate process: | |||
| ⬥ Solid tumor contact with CHA without extension to CA or hepatic artery bifurcation allowing for safe and complete resection and reconstruction | |||
| ⬥ Solid tumor contact with the SMA of ≤180° | |||
| ⬥ Solid tumor contact with variant arterial anatomy (e.g., accessory right hepatic artery, replaced right hepatic artery, replaced CHA, and the origin of replaced or accessory artery) and the presence and degree of tumor contact should be noted if present, as it may affect surgical planning | |||
| ❖ Pancreatic body/tail: | |||
| ⬥ Solid tumor contact with the CA of ≤180° | |||
| Venous: | |||
| ❖ Solid tumor contact with the SMV or PV of >180°, contact of ≤180° with contour irregularity of the vein or thrombosis of the vein but with suitable vessel proximal and distal to the site of involvement allowing for safe and complete resection and vein reconstruction | |||
| ❖ Solid tumor contact with the IVC | |||
| JPS 2019, (16) | BR-PV (SMV/PV invasion alone): no findings of contact and invasion of SMA, CA and CHA. Tumor contact or invasion of the SMV/PV of 180 or more degrees or occlusion of the SMV/PV, not exceeding the inferior border of the duodenum | None | None |
| BR-A (arterial invasion): tumor contact or invasion of SMA and/or CA of less than 180° without showing stenosis or deformity. Tumor contact or invasion of CHA without showing tumor contact or invasion of PHA and/or CA | |||
| Katz et al. 2008, (10) | Tumor abutment (≤180° of the circumference of the vessel) of the SMA or celiac axis | CT findings suspicious for, but not diagnostic of metastatic disease and those with known N1 disease from either prereferral laparotomy or endoscopic ultrasonography guided fine-needle aspiration | Patients with borderline resectable disease owing to a marginal performance status (Zubrod 3), or those with a better performance status and severe preexisting medical comorbidity thought to require protracted evaluation that precluded immediate operation |
| Tumor abutment or encasement (>180° of the circumference of the vessel) of a short segment of the hepatic artery, typically at the origin of the gastroduodenal artery; or short-segment occlusion of the SMV, PV, or SMV-PV confluence that was amenable to vascular resection and reconstruction because of a patent SMV and PV below and above the area of tumor-related occlusion |
PDAC, pancreatic ductal adenocarcinoma; IAP, International Association of Pancreatology; BR, borderline resectable; PV, portal vein; SMV, superior mesenteric vein; SMA, superior mesenteric artery; CA, celiac artery; CHA, common hepatic artery; PHA, proper hepatic artery; PET, positron emission tomography; CT, computed tomography; NCCN, National Comprehensive Cancer Network; SMV, superior mesenteric vein.
With regards to tumor biology, the median serum CA19-9 level was 309.7 U/mL (range, 2.0–12,000.0 U/mL) among all patients. Furthermore, a total of 25 (25.8%) patients had regional lymph node metastasis, as demonstrated by preoperative imaging. Six of these were diagnosed by preoperative PET-CT.
As to conditional factors, 89 (91.8%) patients had a preoperative ECOG score of 0–1, and 8 (8.2%) patients had a preoperative ECOG score of 2. There were no patients with a status score of 3 or more. The median BMI of all patients was 23.63 kg/m2, and 38 (39.2%) patients had weight loss within 3 months prior to diagnosis. Additionally, 27 (27.8%) patients had preoperative jaundice and 47 (48.5%) patients had abdominal pain at presentation. A higher proportion of patients in the IAP-BR group had weight loss within 3 months before surgery, compared to patients in the IAP-R group (46.6% vs. 28.9%; P=0.03). There was no significant difference between the two groups with regards to symptom onset. The majority of patients (57.7%) had a preoperative modified Glasgow prognostic score (m-GPS) of 0, and a higher proportion of patients in the IAP-BR group had a preoperative mGPS of 1 or 2 (36.7% vs. 16.7%).
Pathological findings and outcomes
Overall, 69 (71.1%) patients in this cohort underwent a pancreaticoduodenectomy, including 6 (6.2%) laparoscopic procedures. Additionally, 28 (28.9%) patients underwent distal pancreatectomy and 3 (3.1%) underwent laparoscopic procedures. There were no statistically significant differences in RFS (11.8 vs. 10.3 months; P=0.683) and OS (22.9 vs. 24.0 months; P=0.672) between patients who underwent PD/LPD and DP/LDP.
A total of 70 (72.2%) pancreatic cancer patients received at least one cycle of postoperative adjuvant chemotherapy after undergoing radical resection. Among them, 40 (57.1%) patients received adjuvant chemotherapy for more than 3 months; 18 (25.7%) patients received adjuvant chemotherapy for more than 6 months. Thirty-eighty (54.3%) patients changed their regimen during adjuvant chemotherapy because of disease progression or toxicity intolerance or other reasons. Adjuvant chemotherapy regimens including Gemcitabine (Gem) (n=41), Fluorouracil + Folinic acid + Irinotecan + Oxaliplatin (FOLFIRINOX) (n=10), Gem + Nab-Paclitaxel (n=7), S-1 (n=5), Gem + S-1 (n=4) and Gem + Capecitabine (n=3). There was no significant difference in the rate of adjuvant chemotherapy between the IAP-R and IAP-BR groups (68.4% vs. 78.6%; P>0.05).
With regards to postoperative pathological staging, referring to the 8th edition of the AJCC TNM staging system, most patients had stage I (n=43, 44.3%) and stage II (n=39, 40.2%) disease. There was no significant difference in the postoperative pathological staging between the IAP-R group and the IAP-BR group. However, the T-stage of patients in the IAP-BR group was mostly concentrated within the T2 and T3 stages, while the tumors in the IAP-R group were mostly concentrated in the T1 and T2 stages (P>0.05). Furthermore, the percentage of two or more regional lymph node metastases (N2 stage) was higher in the IAP-BR group (22.0% vs. 5.3%; P<0.05). Consequently, there was a significant difference in the positive lymph node ratio (pLNR) between the two groups (P<0.01). The overall R1 resection rate after undergoing a pancreatectomy was 11.3% in all patients. There were no significant differences observed between the two groups with regards to positive margins (P=0.33), degree of tumor differentiation (P=0.91), and perineural invasion (P=0.85).
With regards to patient outcomes, the median RFS was 18.3 months (95% CI: 10.7–25.9 months) in the IAP-R group, which was significantly superior compared to the IAP-BR group (median RFS: 9.2 months; P<0.01). Additionally, a higher proportion of patients in the IAP-BR group had early recurrence (62.7% vs. 42.1%; P=0.047). Furthermore, a prolonged OS was observed in the IAP-R group (median OS of 30.6 months, 95% CI: 27.0–34.2 months), which was better than 19.1 months (95% CI: 12.6–25.5 months; P<0.05) of the IAP-BR group. The RFS, as well as OS Kaplan-Meier curves of patients in both groups are presented in Figure 1.
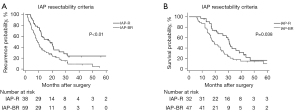
Independent factors associated with RFS and OS of anatomically resectable PDAC
Anatomical factors including maximum tumor diameter (<2.5 vs. ≥2.5 cm), PV/SMV abutment on CT imaging (no vs. yes), biological factors including CA19-9 level (<500 vs. ≥500 U/mL), regional lymph node metastasis on PET-CT or enhanced CT imaging (LNM− vs. LNM+) and conditional factors including ECOG score (≤1 vs. >1), BMI (≥18.5 vs. <18.5 kg/m2), weight loss within 3 months before surgery (no vs. yes), and preoperative mGPS (0 vs. >0) were investigated using Cox-proportional hazards modeling, respectively. Results of univariate and multivariate analyses are presented in Table 3.
Table 3
| Pre-operative factors | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Recurrence-free survival | |||||||
| Tumor size (max) >2.5 cm | 1.74 | 1.10–2.77 | 0.01 | – | – | – | |
| PV/SMV abutment | 2.73 | 1.24–6.02 | 0.01 | 2.30 | 1.14–4.67 | 0.02 | |
| ECOG score >1 | 1.21 | 0.52–2.79 | 0.67 | – | – | – | |
| BMI <18.5 kg/m2 | 1.55 | 0.99–2.45 | 0.38 | – | – | – | |
| Weight loss | 1.66 | 1.03–2.67 | 0.03 | – | – | – | |
| mGPS >0 | 2.54 | 1.37–4.71 | 0.003 | 2.12 | 1.25–3.58 | 0.005 | |
| IAP BR-B | 1.93 | 1.22–3.05 | 0.005 | 2.27 | 1.33–3.87 | 0.003 | |
| CA19-9 >500 U/mL | 1.99 | 1.23–3.25 | 0.005 | – | – | – | |
| LNM (+) | 1.86 | 1.03–3.36 | 0.03 | – | – | – | |
| Overall survival | |||||||
| Tumor size (max) >2.5 cm | 1.76 | 1.11–2.78 | 0.01 | – | – | – | |
| PV/SMV abutment | 2.33 | 0.87–6.23 | 0.001 | 2.89 | 1.37–6.07 | 0.005 | |
| ECOG score >1 | 1.40 | 0.57–3.42 | 0.39 | – | – | – | |
| BMI <18.5 kg/m2 | 1.17 | 0.39–3.45 | 0.76 | – | – | – | |
| Weight loss | 0.99 | 0.63–1.58 | 0.98 | – | – | – | |
| mGPS >0 | 1.74 | 0.89–3.40 | 0.05 | – | – | – | |
| IAP BR-B | 1.59 | 1.01–2.50 | 0.04 | 1.74 | 1.09–2.79 | 0.02 | |
| CA19-9 >500 U/mL | 1.35 | 0.84–2.18 | 0.19 | – | – | – | |
| LNM (+) | 1.65 | 0.90–3.02 | 0.05 | – | – | – | |
PDAC, pancreatic ductal adenocarcinoma; HR, hazard ratio; CI, confidence interval; BR-B, biological borderline resectable; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; mGPS, modified Glasgow prognostic score; IAP, International Association of Pancreatology; PV, portal Vein; SMV, superior mesenteric vein; LNM, lymph node metastasis.
Among the anatomical factors, PV/SMV abutment was found to be independently associated with an increased likelihood of tumor recurrence [hazard ratio (HR): 2.30; P=0.02] and shorter postoperative survival (HR: 2.89; P=0.005) of anatomically resectable PDAC (Figure 2A,2B). Tumor diameter was not a strong prognostic factor.
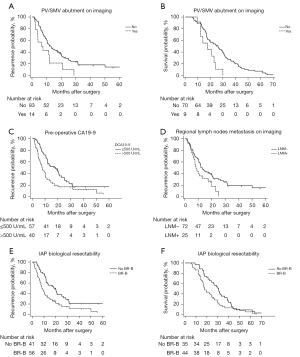
Among all the indicators associated with a patient’s preoperative performance, the ECOG score >1 was not a significant prognostic factor for RFS and OS of anatomically resectable PDAC (RFS: P=0.67; OS: P=0.39), which is not in agreement with the current IAP criteria. Similar results were observed in the univariate analysis results of BMI <18.5 kg/m2 (RFS: P=0.38; OS: P=0.76) and preoperative weight loss (RFS: P=0.03; OS: P=0.98). Notably, the mGPS score >0 was validated as being negatively associated with RFS of anatomically resectable PDAC in multivariate analyses. However, this does not increase the risk of death (P>0.05).
In terms of biological factors, compared to patients without a high-level pre-op CA19-9 and without any imaging evidence of regional LNM, the outcomes were found to be much worse among patients who developed both (median RFS: 19.9 vs. 8.2 months, P<0.001; median OS: 30.6 vs. 17.7 months, P=0.02). This result demonstrates that biological factors play an important role in the resectability assessment of pancreatic cancer, and that BR-B, as defined by IAP, is a significant prognostic factor for both RFS and OS among anatomically resectable PDAC patients (Figure 2C-2F).
Discussion
In 2016, international consensus on a new criterium for BR PDAC was established via the IAP, based on previous studies (17-19). IAP criteria expanded the definition of BRPC from a single anatomical dimension to tumor biology and individual status dimensions (8). This study aimed to investigate the function of biological and conditional factors in the resectability assessment of PDAC, based on the resectability criteria proposed by IAP.
First, consistent with results from previous studies (3,4), tumor recurrence was observed in a total of 69.7% of patients with anatomically resectable pancreatic cancer (NCCN criteria) within 12 months after curative resection in our study. Median OS was 24.0 months among all enrolled patients. The overall 5-year survival rate was 25.9%. Subgroup analysis was conducted according to IAP-criteria. Although there were no significant differences in the rate of postoperative adjuvant chemotherapy between the two groups, the RFS and OS of IAP-BR group were still significantly worse, compared to the IAP-R group. Furthermore, there was a higher rate of early recurrence (<12 months), and pathologically confirmed lymph node metastasis in the IAP-BR group. These findings suggest that PDAC patients who were at high post-operative risk of early recurrence and metastasis could be identified by IAP-criteria. These patients were more likely to benefit from neoadjuvant therapy, rather than upfront surgery.
The BR-B PDAC was defined as an anatomical potentially resectable tumor with suspicious distant metastasis. CA19-9 values at presentation were utilized to evaluate tumor biological behavior in IAP-criteria (8). Hartwig et al. (17) discovered that elevated preoperative CA19-9 levels were found to be closely associated with reduced resectability and survival of pancreatic cancer. When serum CA19-9 >500 U/mL, the R0 resection rate was found to be less than 40% among resectable patients, the overall 5-year survival rate was <7%, and the median survival time of <15.4 months. Based on these results, the preoperative serum CA19-9 ≥500 U/mL was recommended by an IAP expert panel as one of the diagnostic criteria for biologically resectable PDAC. In our study, pre-operative CA19-9 >500 U/mL was found to be equivocally associated with tumor recurrence in anatomically resectable PDAC, and these patients have a median RFS of only 7.5 months. This indicates that a tumor with significantly elevated preoperative CA19-9 is highly aggressive. Bergquist et al. (20) reviewed 28,074 PDAC patients with reported CA19-9 expression in the National Cancer Data Base (NCDB 2010 to 2012), and found that elevated CA19-9 (>37 U/mL) in anatomically resectable PDAC is significantly associated with decreased OS. Kato et al. (13) analyzed 369 PDAC patients who underwent upfront surgery, and proposed that CA19-9 >1,000 U/mL is a more reasonable cut-off value to define biological BR-PDAC. However, all relevant studies, including the present study, were conducted in a retrospective cohort, and the cut-off value of pre-operative CA19-9 could be affected by different sample sizes. Furthermore, analysis of serological indices also varies depending on each patient’s preoperative serum bilirubin level and Level and Lewis A−B− gene expression (20). Therefore, further prospective studies are highly awaited to determine a justified cut-off value of CA19-9.
In addition to CA19-9 levels, the IAP-criteria also includes regional lymph node status as one of the important predictors to assess the biological resectability of PDAC. The rationale was that LNM independently affects prognosis of PDAC patients according to previous study, regardless of T staging and anatomical resectability (18). However, the diagnosis of preoperative LNM, proposed by the IAP consensus, requires PET-CT examination or lymph node biopsy. Therefore, it cannot be used routinely in practice. Kato et al. (13) discovered that contrast-enhanced abdominal CT scans have a similar specificity (84% vs. 90%, P=0.734), sensitivity (33% vs. 30%; P=0.844) and positive prediction rate (81% vs. 83%; P=0.100), compared to PET-CT on diagnosis of LNM in their cohort. The present study validates that LNM suggested by preoperative enhanced CT/PET-CT is an independent prognostic factor for both RFS and OS of anatomically resectable PDAC patients. It plays an important role in the biological resectability assessment of PDAC.
Another recommendation by IAP criteria for resectability assessment of PDAC is the patient’s condition before surgery, which is closely related to the patient’s surgical tolerance, post-operative complication rate and OS. A retrospective study conducted by Tas et al. (19) discovered that a preoperative ECOG score ≥2 was independently associated with decreased OS across all tumor stages. In the IAP consensus, an ECOG score of ≥2 is also utilized as the cut-off point to differentiate between resectable and BR-C tumors. However, in the present study, ECOG score >1 was not a significant prognostic factor for RFS and OS of anatomically resectable PDAC. Nevertheless, this result should be interpreted with caution. First, only eight patients had a preoperative ECOG score equal to 2 in our study, and there were no patients with a score >2. On the other hand, different from anatomical and tumor biological factors, ECOG scores are comparatively subjective and can be improved through a preoperative multidisciplinary treatment, nutritional support, and other rehabilitative therapies. It has been suggested that patients’ systemic inflammatory response and nutritional status may be objective conditional factors that need to be considered when determining tumor resectability. This is because some indicators related to patients’ immune and nutritional status, such as m-GPS, neutrophil lymphocyte ratio (NLR) (21), and Controlling Nutrition Status (CONUT) score (13), have been reported to adversely affect the patients’ prognosis. The m-GPS >0 was validated to be an independent factor associated with decreased RFS of anatomically resectable PDAC in present study. These results suggest that anatomical resectable PDAC patients with elevated preoperative CRP and reduced serum albumin do not benefit from upfront surgery.
In addition, this study focused on the significance of anatomical factors with regards to the assessment of resectability in patients with pancreatic cancer. When tumor contact with PV/SMV was less than 180°, and there were no venous contour irregularities on CT imaging, both NCCN and IAP anatomical resectable criteria were met, the risk of recurrence and death after radical surgery was still significantly increased. Whether such patients were able to benefit from neoadjuvant therapy remains to be answered by results from prospective studies in the future.
In fact, there is still a great heterogeneity of BR definition of PDAC can be observed in different guidelines and criteria at present (Table 2). From the anatomical dimension, for example, many different terms (“abutment”, “encasement” and “occlusion”) can lead to biased interpretations (22) and cross-sectional comparison of different research findings becomes difficult. Although the IAP criteria have been proposed for many years, biological and conditional criteria of BRPC is still not widely adopted by the clinical practice (15,16). An increasing number of studies have begun to focus on the importance of biological and conditional factors on the resectability of pancreatic cancer, however, most studies, including present study, are limited to a retrospective design or a relatively small sample size. More prospective studies are required to clarify the controversial issues on IAP-criteria and promote its transformation from an expert consensus to a widely accepted clinical practice guideline.
In summary, the IAP-criteria marked the way forward for future studies, and it is justified to evaluate the resectability of pancreatic cancer, combined with anatomical and biological factors according to IAP criteria. Whether conditional factors should be included in the resectability criteria needs to be validated by prospective and large cohorts.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1258/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1258/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1258/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1258/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University (No. 2020-506) and informed consent exemption had been obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 2019;16:11-26. [Crossref] [PubMed]
- Khorana AA, Mangu PB, Berlin J, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2541-56. [Crossref] [PubMed]
- Groot VP, Rezaee N, Wu W, et al. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg 2018;267:936-45. [Crossref] [PubMed]
- Jones RP, Psarelli EE, Jackson R, et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg 2019;154:1038-48. [Crossref] [PubMed]
- Groot VP, Gemenetzis G, Blair AB, et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg 2019;269:1154-62. [Crossref] [PubMed]
- Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell 2012;148:21-3. [Crossref] [PubMed]
- Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012;148:349-61. [Crossref] [PubMed]
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11. [Crossref] [PubMed]
- Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001;5:27-35. [Crossref] [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 846-8. [Crossref] [PubMed]
- Anger F, Döring A, van Dam J, et al. Impact of Borderline Resectability in Pancreatic Head Cancer on Patient Survival: Biology Matters According to the New International Consensus Criteria. Ann Surg Oncol 2021;28:2325-36. [Crossref] [PubMed]
- Hayasaki A, Isaji S, Kishiwada M, et al. Survival Analysis in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Chemoradiotherapy Followed by Surgery According to the International Consensus on the 2017 Definition of Borderline Resectable Cancer. Cancers (Basel) 2018;10:65. [Crossref] [PubMed]
- Kato Y, Yamada S, Tashiro M, et al. Biological and conditional factors should be included when defining criteria for resectability for patients with pancreatic cancer. HPB (Oxford) 2019;21:1211-8. [Crossref] [PubMed]
- Imrie CW. Host systemic inflammatory response influences outcome in pancreatic cancer. Pancreatology 2015;15:327-30. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Pancreatic Adenocarcinoma. Version 1. 2022.
- Japan Pancreas Society. General rules for the study of pancreatic cancer. 7th edition. Tokyo: Kanehara & Co., Ltd.; 2019.
- Hartwig W, Strobel O, Hinz U, et al. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol 2013;20:2188-96. [Crossref] [PubMed]
- Isaji S, Kishiwada M, Kato H. Surgery for Borderline Resectable Pancreatic Cancer: The Japanese Experience. In: Sa K M a A, editor. Multimodality Management of Borderline Resectable Pancreatic Cancer. Switzerland: Springer International Publishing, 2016:265-87.
- Tas F, Sen F, Odabas H, et al. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol 2013;18:839-46. [Crossref] [PubMed]
- Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J Am Coll Surg 2016;223:52-65. [Crossref] [PubMed]
- Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 2013;109:416-21. [Crossref] [PubMed]
- Nappo G, Donisi G, Zerbi A. Borderline resectable pancreatic cancer: Certainties and controversies. World J Gastrointest Surg 2021;13:516-28. [Crossref] [PubMed]


