
Trastuzumab treatment of invasive breast ductal carcinoma induces severe edema: a case report
Introduction
Overexpression of human epidermal growth factor receptor 2 (HER2) is widely reported in breast cancer patients and represents a clinically relevant biomarker for its treatment (1). Trastuzumab, a monoclonal antibody which binds to the extracellular domain of HER2, is the first biological drug approved for the treatment of HER2-positive breast cancer. However, trastuzumab also exhibits a series of clinical adverse effects, including cardiac toxicity, nerve damage, edema, abnormal liver function, thrombocytopenia, etc. In this case report, we report that a 46-year-old female patient with invasive ductal carcinoma developed a rare severe edema in neck, face, chest, abdomen and both upper limbs after single dose trastuzumab treatment, supporting the notion that potential allergic reaction of trastuzumab should be concerned in clinical application. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1607/rc).
Case presentation
A 46-year-old female underwent surgery on April 20th, 2020, and was diagnosed as invasive ductal carcinoma on the right side. Histopathology analysis confirmed a massive infiltration of inflammatory cells in the interstitium. In detail: the size of the mass was 2.5 cm × 1.3 cm, while no clear nerve invasion was observed. Immunostaining of tissue section showed the levels and distribution of various tumor markers, including Ki67 (~70%+), AR (~80%+), P53 (~95%+), ER (~70%+), PR (<1%), HER2 (3+), EGFR (−), P63 (+), calponin (+), EMA (+). The tumor was classified as Stage 2B. After surgery, the patient was administered with 1 cycle of paclitaxel combined with cyclophosphamide and doxorubicin treatment on May 12th. However, as paclitaxel caused significant hair loss in this patient, the chemotherapy was adjusted. The patient received 2 cycles of docetaxel and epirubicin combined with cyclophosphamide therapy on June 2nd, 2020 and June 23th, 2020. After three chemotherapy sessions, a single dose of trastuzumab (440 mg) was administrated on June 25th. After two days, the patient developed edema symptoms. However, she did not notice and take any medication. The edema gradually worsened until July 15th, 2020. As shown in Figure 1, the patient had developed severe edema in the neck, face, chest, abdomen and both upper limbs. The hands of the patient presented the most remarkable edema symptoms, in which the epidermis was transparent and tight (Figure 2). The skin temperature was high. Moreover, the patient had obvious pharyngeal edema and could only take a small amount of fluid diet. The patient was also unable to stand and walk due to motor and sensory nerve damage in both upper and lower extremities induced by trastuzumab administration. Moreover, the patient developed an immunocompromised state and was susceptible to pulmonary infection.
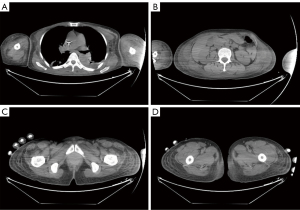
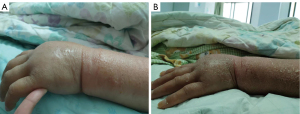
Due to such severe situation, our hospital carried out whole-hospital consultation, and prestigious dermatologists from other hospitals were invited to participate in the consultation. Following careful examination, physicians and specialists considered the edema of the patient with unknown origin and excluded angioedema. Due to her allergy to iodine contrast media, enhanced CT examination of edema could not be performed. In addition, due to the severe edema, biopsy could not be performed to further clarify the etiology. However, from August 15th, 2020, 20 days after administration of trastuzumab, the patient was administered with methylprednisone 80 mg per day for 5 days. The edema in neck, face and both upper limbs of the patient was mildly reduced, though the follow-up CT showed no significant reduction in edema. As shown in Figure 2, the hands of the patient not only displayed a significantly decrease of skin tightness, but also enhancement of desquamated epidermis and skin pigmentation. However, the nerve sensitivity of both upper limbs and lower limbs was improved. Particularly, her lower limbs gained tactile sensation and improved muscle strength. The patient was able to stand up but unable to walk. She could not eat regular food but only liquid diet.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. This study is approved by Ethic Committee of Jinling Hospital, Nanjing University School of Medicine, and signed consent form was obtained from the patient (No. 81YY-KYLL-11-01).
Discussion
Up to 15–20% of early breast cancer exhibits overexpression or amplification of the HER2 receptor (2). Trastuzumab has a major effect in reducing tumor recurrence and death in patients with such type of early breast cancer (3). Although there are other targeting HER2 agents, such as pertuzumab, currently available for patients with HER2-positive breast cancer, trastuzumab remains as the gold standard for treatment of this disease subtypes. Accumulating evidences have shown that trastuzumab plays a critical role in immune regulation in order to achieve an anti-tumor efficacy. Treatment with trastuzumab can either induce immune-mediated anti-tumor effect or increase the possibility of synergistic regulation of immune system. In addition, trastuzumab combined with chemotherapy can enhance chemotherapy-induced tumor cytotoxicity via decreasing DNA repair activity and increasing cell apoptosis (4). Chen et al. (4) reported that when trastuzumab was applied in conjunction with adjuvant chemotherapy, the advantage of 1-year treatment was more significant.
Nonetheless, trastuzumab also exhibited certain side effects, mainly manifested as heart damage. Peripheral edema and lymphedema are commonly observed, but mainly exhibit as local edema. To our knowledge, no case of diffuse edema in multiple parts of the body has been reported previously. We postulate that the severe edema in this patient is likely related to the trastuzumab-induced allergic reaction, which results in endothelial cell damage and inflammatory cytokines production. It may cause reduction in vascular wall integrity, increment of vascular permeability and aberrant hemorheology. Subsequently, the blood flows into peripheral tissues, leading to edema (5). Since allergies can increase energy consumption, insufficient protein intake may further aggravate the edema. Another possibility is that membrane rupture and protein exudation into the infarct area collectively increase cell permeability, which leads to regional blood flow suppression and irreversible tissue damage (6). In addition, allergic reaction can further aggravate oxidative stress, leading to upregulation of angiotensin II, which in turn results in increase of inhibitory signals and oxidative stress (1). In this case report, we found that after 5 days of treatment with methylprednisone, the edema in neck, face and both upper limbs of the patient were mildly reduced. Glucocorticoid has anti-inflammatory, anti-edema, anti-allergy and management cancer-related symptoms (compression, pain, edema, etc.) (7). This result argues that if hormone therapy is given at the early stage, the patient may not experience severe edema later on.
In summary, our report suggests that trastuzumab administration in breast cancers may cause severe allergic reactions, which should be concerned in clinical application. Individual patient may have different sensitivities to drugs, and the occurrence of adverse reactions are different. In particular, patients who have an allergic constitution should pay more attention to the adverse reactions. In clinical application, the early-stage adverse reactions induced by drug administration should be carefully considered in order to avoid the potential serious adverse effects.
Acknowledgments
We greatly appreciate Ms. Jane Zen (Wellesley College, Boston, MA, USA) for her help in polishing our paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1607/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1607/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1607/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. This study is approved by Ethic Committee of Jinling Hospital, Nanjing University School of Medicine, and signed consent form was obtained from the patient (No. 81YY-KYLL-11-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nicolazzi MA, Carnicelli A, Fuorlo M, et al. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur Rev Med Pharmacol Sci 2018;22:2175-85. [PubMed]
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195-205. [Crossref] [PubMed]
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 2017;377:122-31. [Crossref] [PubMed]
- Chen L, Zhou W, Hu X, et al. Short-duration versus 1-year adjuvant trastuzumab in early HER2 positive breast cancer: A meta-analysis of randomized controlled trials. Cancer Treat Rev 2019;75:12-9. [Crossref] [PubMed]
- Semeraro F, Cancarini A, dell'Omo R, et al. Diabetic Retinopathy: Vascular and Inflammatory Disease. J Diabetes Res 2015;2015:582060. [Crossref] [PubMed]
- Nag S, Manias JL, Stewart DJ. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol 2009;118:197-217. [Crossref] [PubMed]
- Kalfeist L, Galland L, Ledys F, et al. Impact of Glucocorticoid Use in Oncology in the Immunotherapy Era. Cells 2022;11:770. [Crossref] [PubMed]


