
Mast cell chymase is in contact with melanoma cells in vivo and it detaches melanoma cells from the substratum in vitro
Introduction
Mast cells are versatile immune cells well-known for their effects in type I hypersensitivity reactions, but they are also involved in many diseases related to ultraviolet (UV) radiation beyond allergy [reviewed in (1)]. In fact, mast cell numbers are higher in sun-exposed than in sun-protected skin (2,3), suggesting a potent role for these cells in skin photobiology.
Melanoma, the rapidly increasing skin cancer (4,5) with UV exposure as its main risk factor (6), has also been studied in regard to mast cells [recently reviewed in (7)]. The proposed effects of mast cells in melanoma have been variable. Increased numbers of mast cells correlating with microvessel density have suggested an enhancing role in angiogenesis (8-10). Higher prevalence of mast cells has been reported in sun-protected skin of melanoma patients compared to controls (11). In addition, data from patients with mast cell activation syndrome suggest that mast cells may promote the development of certain solid tumors, including melanoma (12). In contrast to these findings, others have shown decreased numbers of mast cells in melanomas compared to benign nevi (13,14). In our previous study, low numbers of chymase+ mast cells associated with microsatellites in deeply invasive melanomas, while low numbers of tryptase+ mast cells associated with poor survival and a more advanced tumor stage (15). In line with our results, lower distribution of mast cells was recently observed in higher stages of tumor depth in melanoma, also suggesting an inhibitory effect of mast cells in melanoma progression (16).
Cutaneous mast cells contain predominantly both chymase and tryptase in their secretory granules. Mast cell chymase is a potent chymotryptic serine proteinase that appears to have profound detrimental effects on the cell adherence and migration of normal keratinocytes as well as squamous carcinoma cells of the uterine cervix, though unlike normal keratinocytes the carcinoma cells can be left viable after chymase treatment (17,18). These characteristics of chymase suggest that it may be related to the migration, adherence and spreading of melanoma cells as well. However, chymase is known to be a subject to regulation by a variety of protease inhibitors (17,18), which may determine its net biological effect.
There are no previous studies that have investigated the interaction between mast cell chymase and melanoma cells. It is unclear whether the effect of chymase in melanoma is antitumorigenic or protumorigenic. Because low numbers of chymase+ mast cells associated with microsatellites in deeply invasive melanomas in our previous study (15), the finding prompted to investigate the effects of recombinant human (rh)-chymase on the detachment, migration, proliferation and viability of two different primary melanoma cell lines. In addition, the ability of sonicate extracts from these melanoma cell lines to affect the enzyme activity of rh-chymase was studied. Furthermore, the previous melanoma material (15) was studied in more detail to find out whether immunoreactive chymase can be found in morphological contact with melanoma cells. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1688/rc).
Methods
Microscopy of melanocytic lesions
The 151 tissue samples consisting of 48 deeply (>4 mm) and 39 superficially (<1 mm) invasive melanomas, 14 in situ melanomas, 25 benign and 25 dysplastic nevi, collected at the Kuopio University Hospital as described with detailed clinico-pathological data in our previous study (15) were analyzed for apparent cell-to-cell contacts between chymase+ mast cells and melanocytic cells. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Kuopio University Hospital and by the Finnish National Supervisory Authority for Welfare and Health (No. 71/2017). The registry study protocol was retrospective and thus the consent of the patients for participation was not required. The number of contacts was estimated semi-quantitatively by using a scale from 0 to 3 (0= no contacts, 1= sporadic contacts, 2= few contacts, 3= many contacts).
Cell culture
WM115 cells originating from a primary melanoma (received as a generous gift from Piia Takabe, MSc, and Docent Sanna Pasonen-Seppänen, PhD, Institute of Biomedicine, University of Eastern Finland) were cultured in High Glucose DMEM (PAA Cell Culture Company, Cambridge, UK) supplemented with 5 % inactivated fetal bovine serum (FBS) (PAA Cell Culture Company), 2 mM glutamine (Lonza, Basel, Switzerland), 1 mM sodium pyruvate (Thermo Fisher Scientific, Waltham, MA, USA) and 50 U/mL penicillin – 50 µg/mL streptomycin sulfate (Lonza). Cells were passaged twice a week at a 1:10 split ratio using 0.02% trypsin (w/v) - 0.02% EDTA (w/v) (Lonza).
G361 cells originating from a primary melanoma (Sigma-Aldrich, Munich, Germany) were cultured in McCoy’s 5a medium (Sigma-Aldrich, order No. 88030401) supplemented with 2 mM glutamine (Lonza) and 10% FBS (PAA Cell Culture Company). Cells were passaged twice a week at a 1:10 split ratio using 0.02% trypsin (w/v) - 0.02% EDTA (w/v) (Lonza).
Chemicals
Rh-chymase dissolved in 20 mM Tris-HCl – 0.8 M NaCl – 25% glycerol (pH 7.6), chymostatin dissolved in 100% DMSO, heparin glycosaminoglycan from porcine intestinal mucosa dissolved in deionized H20, type I collagen, heparinase dissolved in 20 mM Tris-HCl, 4 mM CaCl2 and 0.01% BSA in 50 mM NaCl, aprotinin dissolved in deionized H20, substance P dissolved in DMSO, phorbol 12-myristate 13-acetate (PMA) dissolved in DMSO and A23187 dissolved in DMSO were all from Sigma-Aldrich. Acetic acid was from Fluka BioChemica (Buchs, Germany). Suc-Ala-Ala-Pro-Phe-p-nitroanilide dissolved in DMSO as a 20 mM stock solution was purchased from Bachem (Bubendorf, Switzerland).
Cell detachment
To investigate the concentration of rh-chymase needed to detach melanoma cells from cell culture plates, the cells were plated inside of 6 mm metallic sylinders on the wells of an uncoated 24-well plate (Falcon, Becton–Dickinson, Plymouth, UK) as earlier described in our previous studies (17,18). Cell detachment was also investigated from plates precoated with 50 µg/mL type I collagen (diluted in 0.02 M acetic acid) for 1 h and then washed 3 times with PBS prior to use. After equilibration for 1 h at 37 ℃ and 5% CO2 in 0.4 mL of complete DMEM medium, about 30,000 melanoma cells were added cautiously into each cylinder. The cells were allowed to adhere onto the plastic surface overnight and thus until complete confluence of cells. Next, the metallic cylinders were removed, the wells were carefully washed twice with PBS and the medium was changed to DMEM without FBS. After equilibration for 1 h, rh-chymase, heparin, heparinase and/or chymostatin were added in varying combinations and concentrations as described in Results. The cells were cultured overnight and, thereafter, the medium was changed to 4% formaldehyde for 2 h for fixing the cells. To visualize the cells, Mayer’s hematoxylin staining overnight was performed. The experiments were repeated 3–4 times. The concentration of chymase was chosen by preliminary testing and data from (18).
Migration
To investigate the effects of rh-chymase on melanoma cell migration, the wells of a 24-well plate (Falcon) were first coated with 50 µg/mL type I collagen (diluted in 0.02 M acetic acid) for 1 h in room temperature and then washed three times with PBS. Thereafter, 70,000 melanoma cells were added to the wells in complete medium and cultured overnight to reach confluence. Next, the medium was changed to serum-free medium containing 0–0.01 µg/mL of rh-chymase and/or 0–20 µg/mL of chymostatin and the cells were cultured overnight. On the next morning, two lines (~1-mm wide) crossing each other at perpendicular angles were drawn with a 200-µL disposable pipette tip. The migration of cells to the cleared area was inspected under a microscope. The experiments were repeated 3–4 times. The areas covered with cells were measured before and 6 h after the treatment. The change in the area was counted in pixels using the NIH ImageJ software (available at https://imagej.nih.gov/ij/) and converted to mean migration distance (µm) of the cell front. The concentrations of chymase for migration experiments was chosen based on the concentrations used in the cell detachment experiments to enable cell adherence to culture plates.
Proliferation
Melanoma cells (3,000 cells/well in quadruplicate wells) were plated in a 96-well plate in 0.2 mL of complete medium. On the following day, the medium was changed to basal medium. On the third day after the plating, the medium was changed to fresh medium without serum, and the cultures were treated overnight with rh-chymase and/or chymostatin at final concentrations of 0.001–0.01 µg/mL for chymase (same as in migration) and 20 µg/mL for chymostatin. On the fourth day after the plating, 3H-thymidine (15.5 Ci/mmol, Perkin-Elmer, Turku, Finland) was added to each well and the cultures were incubated overnight. The wells were then washed 3 times with cold PBS and once with cold 6% trichloroacetic acid, and the cells were solubilized with 0.1 M NaOH containing 1% sodium dodecyl sulfate. Solubilized radioactivity was counted in a LKB 1215 Rackbeta liquid scintillation counter (Wallac, Turku, Finland) (19). At the end of the incubation period, the cell cultures had not reached confluence. The experiment was done in dublicate wells and repeated three times. The proliferation of cells could have been measured also in a cell suspension for example by analyzing DNA synthesis, but it would have been nonphysiological to analyze the proliferation of adherent cells in a cell suspension.
Cell viability
Melanoma cells (60,000 cells/well) were seeded in 0.4 mL of complete medium in a 24-well plate and cultured overnight until about 70% confluence. The next day, the wells were washed twice with basal medium in which the cells were then allowed to equilibrate for 1 h before addition of 1 or 5 µg/mL rh-chymase or its diluent control to the cells. The following day, the viability of the detached cells was estimated after staining with Trypan Blue (Sigma). Control cells treated with diluent control only were detached with a short incubation with trypsin-EDTA (18). Thereafter, the detached cells were cultured in a new plate in complete medium and their growth was inspected under a microscope. The experiment was done in dublicate wells and repeated three times. The concentration of chymase was chosen by preliminary testing and data from (18).
Effect of melanoma cell lysate on the enzyme activity of rh-chymase
The effect of melanoma cell sonicates on the enzyme activity of rh-chymase was tested as described in detail in our previous study (18). Briefly, melanoma cell sonicates were obtained with an ultrasound device, Labsonic U-2000 ultrasonic homogenizer (B. Braun Diessel Biotech GmbH, Melsungen, Germany), in an ice bath. Thereafter, the microcentrifuged supernatant of the cell sonicate was tested for its capability of inhibiting rh-chymase activity by using 0.2 mM Suc-Ala-Ala-Pro-Phe-pNA as the substrate, 0.5 mg/mL aprotinin as the inhibitor of possible background activity, and 100 mM Tris–HCl buffer, pH 7.5, containing 1.5 M NaCl. Chymase activity was measured spectrophotometrically on a microplate reader (Tecan Sunrise, Tecan Group Ltd., Männedorf, Switzerland) at the wavelength of 405 nm. The experiment was done in dublicate wells and repeated three times.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD) or standard error (SE). For comparisons of the means between different treatment groups, repeated measures ANOVA with Tukey’s post test was performed to test statistical significance (P<0.05). To analyze the cell-to-cell contacts between chymase+ mast cells and melanocytic cells in different melanocytic lesions and lymph node metastases, Kruskal-Wallis test with Dunn’s multiple comparisons tests was performed. GraphPad Instat version 3.05 (GraphPad Software Inc., San Diego, CA, USA) was used in all analyses.
Results
Morphological contacts between melanocytic cells and chymase+ mast cells
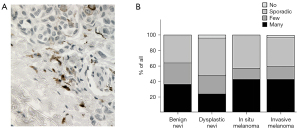
Benign and malignant melanocytic lesions were analyzed semi-quantitatively for apparent cell-to-cell contacts between melanocytic cells and chymase+ mast cells (Figure 1A). Chymase is located in the secretory granules of mast cells and therefore diffuses to the surroundings from the site of degranulated mast cell. In deeply invasive melanomas, 39.6% of the samples showed many contacts between chymase+ mast cells and melanoma cells (Figure 1B). The corresponding percentages were 46.2% and 42.9% for superficially invasive and in situ melanomas, respectively. Only 36% of benign nevi and 24% of dysplastic nevi showed similar contacts. In summary, these data show that mast cells and melanoma cells are in contact during melanoma progression. More contacts were found in malignant melanomas compared to benign and dysplastic nevi.
Both melanoma cell lines are detached from the plastic surface by rh-chymase
Both cell lines, WM115 and G361, were detached completely from noncoated wells at already 0.1 µg/mL rh-chymase (Figure 2). Chymostatin, a strong inhibitor of several chymotryptic proteinases including chymase, inhibited the cell detachment at both 10 and 20 µg/mL concentrations. In both cell lines studied, addition of 10 µg/mL heparin glycosaminoglycan in the reaction hindered the cell detachment.
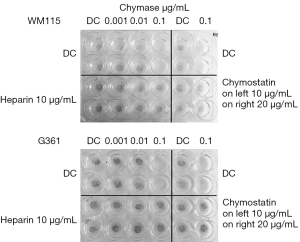
In collagen-coated wells, the WM115 cells were detached at 0.1 µg/mL rh-chymase in the presence of heparinase suggesting an adherence-enhancing role for heparan sulphate onto collagen, but were not detached in the absence of heparin or heparinase (Figure 3). Interestingly, the WM115 cells were totally detached by rh-chymase in the presence of 1 µg/mL heparin, partially detached in the presence of 3 µg/mL heparin and not detached in the presence of 10 µg/mL heparin. The G361 cells were detached partially already at 0.01 µg/mL rh-chymase and even more so in the presence of heparinase, while the detachment was complete at 0.1 µg/mL rh-chymase without an effect of heparinase. At 0.01 µg/mL and partially also so at 0.1 µg/mL rh-chymase, heparin was able to inhibit the detachment of G361 cells.
Rh-chymase decreased the migration of WM115, but not G361 cells
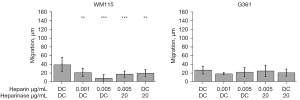
Next, the effect of rh-chymase on migration of melanoma cells on collagen-coated plastic surface was investigated. To prevent cell detachment by rh-chymase during the migration experiment, rh-chymase was diluted 1:20–1:100 of the concentration needed to completely detach melanoma cells as found out in the cell detachment experiments described above. Interestingly, rh-chymase at 0.001 and 0.005 µg/mL decreased the migration of WM115 cells at the 6-h time point (Figure 4). Chymostatin used at 20 µg/mL partially blocked the effect of rh-chymase, but also decreased migration when used alone. Rh-chymase failed to have any significant effect on the migration of G361 cells.
Rh-chymase decreased the proliferation of WM115, but not G361 cells
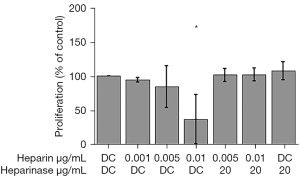
Proliferation of melanoma cell lines was investigated at different concentrations (0.001–0.01 µg/mL) of rh-chymase. Proliferation of WM115 cells was reduced to 36% compared to control (P<0.05) when the cells were treated with 0.01 µg/mL rh-chymase, while 20 µg/mL chymostatin counteracted this effect (Figure 5). Different concentrations (0.001–0.01 µg/mL) of rh-chymase did not have a statistically significant effect on the proliferation of G361 cells (data not shown).
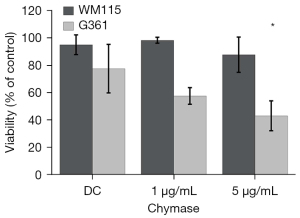
Cell viability
The melanoma cells were completely detached from the plastic surface by culturing them with 1 or 5 µg/mL rh-chymase overnight. The trypan blue-exclusion method showed that more than 98% of WM115 cells and 57% of G361 cells were viable after the 1 µg/mL rh-chymase treatment, while after 5 µg/mL rh-chymase treatment the viability of WM115 and G361 cells was 87% and 43%, respectively (Figure 6). The reduction in viability of G361 cells when treated with 5 µg/mL rh-chymase was significant compared to control cells. Control cells were cultured for the same time period but detached with a short trypsin treatment, showed no significant reduction in the viability of WM115 cells, whereas G361 cells showed only 78% viability. Interestingly, when the cells detached by rh-chymase were re-seeded on a new plate in a fresh, complete medium, WM115 cells grew in a net-like pattern and G361 cells grew in small nodules. In both cell lines, the control cells showed a normal, even growth pattern after re-seeding. The results indicate that the cell viability in detached G361 cells is reduced but not in detached WM115 cells after prolonged rh-chymase treatment.
Effect of melanoma cell lysate on chymase activity
To study the potential of melanoma cells to inactivate rh-chymase, melanoma cell sonicates was tested for its capability of inhibiting the activity of rh-chymase. As a result, no significant inactivation of rh-chymase could be detected (Figure 7). The melanoma cell sonicate itself showed only weak if any chymotryptic activity in the assay conditions used for rh-chymase. Interestingly, there was a slight, although statistically nonsignificant trend towards increasing enzyme activity in G361 cells, which may be explained by a slight ability of the melanoma cell sonicate itself to cause hydrolysis of Suc-Ala-Ala-Pro-Phe-pNA.
Discussion
The present results show the variable effects of rh-chymase on two different primary melanoma cell lines. The WM115 and G361 cells were detached at a very low rh-chymase concentration (0.1 µg/mL) from the noncoated plastic surface. Heparin consistently inhibited cell detachment, which may be due to steric hindrance between cell and plastic surfaces, as heparin can bind chymase resulting in large chymase-heparin molecular complex (20).
The concentrations of rh-chymase used in the present study can´t be directly compared to earlier reports on mast cell densities and chymase concentrations in the skin. In normal skin, the density of mast cells varies from approximately 20 to 50 mast cells/mm2, but is not affected by age or gender (21). The chymase amount in adult foreskin has been quantitated to 4.5 µg/106 mast cells (22). Based on these numbers, the concentrations used in the present study were much lower. However, the chymase concentration in the melanoma microenvironment may be altered because the mast cell density in melanoma has been shown to be different than in normal skin, with both decreased (13-15) and increased (8-10) numbers reported. In addition, the degranulation level of mast cells can determine the level of released chymase.
Cell-surface heparan sulphate proteoglycans have been shown to support the integrin-mediated adhesion to collagen (23). G361 cells were detached effectively by rh-chymase from collagen-coated surfaces, and the detachment was inhibited by heparin. However, heparinase enhanced the detachment from collagen. Thus, it is possible that heparin caused steric hindrance in a complex with rh-chymase, but the role of cell-surface heparan sulphate is prominent. Rh-chymase is not expected to break collagen, but rather enhances the formation of collagen fibrils (24), thus excluding the possibility that cell detachment was due only to breakdown of collagen required for cell attachment on the plastic surface.
Heparinase enhanced the detachment of WM115 cells from collagen, as was the case also in G361 cells, suggesting, again, a role for heparan sulphate. The effect of heparin on the detachment of WM115 cells from collagen by rh-chymase appears to be complex as a detachment-promoting role was noted at low but inhibitory one at high heparin concentration. Heparin is known to form large complexes (Mr 400–560 kDa) with chymase (20) and to modify the enzymatic properties of chymase (25,26), which can explain the variation in chymase effects in our results in regard to the presence or absence of heparin. Accordingly, heparin has been shown to affect the catalytic properties of chymase on complement C3 (27) and fibrinogen (28).
The present results show that the detached cells, even after a prolonged incubation with a high concentration (1 or 5 µg/mL) of rh-chymase, were viable and able to grow in complete medium after re-seeding. Melanoma cells also did not show any inhibitory effect on rh-chymase activity, at least in in vitro conditions. This is in line with findings from SiHa SCC cells (18). Interestingly, the viability of G361 cells was decreased to some extent after prolonged treatment with rh-chymase. Earlier, chymase has been shown to induce apoptosis in several different cell types, such as normal keratinocytes (18), smooth muscle cells (29,30), epithelial cells (31) and endothelial cells (32). One possibility is, that it is due to the specific type of apoptosis, that is, anoikis in these cells.
Although the cells were viable after prolonged treatment with high concentrations of rh-chymase, the growth patterns of WM115 and G361 cells were noticeably different than in control cells after re-seeding in complete medium. This may reflect epithelial-to-mesenchymal transition (EMT) of melanoma cells in response to rh-chymase. Mast cells have been shown to participate in EMT in thyroid cancer (33) and in a mouse model of melanoma metastasis to the lungs (34).
Interestingly, we found significantly reduced migration and proliferation of WM115 melanoma cells when treated with rh-chymase, while no effect was found in G361 cells. Moreover, the migration distance of the nontreated control cells was about 20 µm in G361 cells and 40 µm in WM115 cells. It may be that the G361 cells move only a little in general or would have required a special stimulus to induce migration, and that explains why rh-chymase had no additional effect on the low migration rate of these cells. It is of note that rh-chymase did not show any stimulatory effect on the migration or proliferation in either cell lines. In both cell lines, we noticed a decrease, although not statistically significant, in the migration when the cells were treated with chymostatin only. It is well known that chymostatin is not a chymase-specific inhibitor, explaining our results.
In mouse models, mast cells have been regarded important for migration in intestinal cells (35) and chymase activation is associated with migration also at the wound edge (36). However, mast cells are reduced at the wound edge in human skin (37) and may be harmful to epithelium (17). Regarding our previous finding on the reduced chymase+ mast cell counts associating with increased microsatellites in melanoma (15), it can be hypothetized that if there were enough chymase-producing mast cells, chymase would inhibit migration, proliferation and/or viability of melanoma cells in the tissue and thus reduce the formation of microsatellites.
The consistent finding of this study is that rh-chymase can effectively detach primary tumor melanoma cells from the plastic surface in vitro and chymase+ mast cells can be found in apparent morphological contact with melanoma cells in vivo. It is noteworthy, that the contacts between epidermis and dermal mast cells are rare in normal skin. Our results also show the variable effects of rh-chymase on primary melanoma cells in vitro depending on the cell line, the concentration of rh-chymase, and the presence of regulatory heparin. These results emphasize the heterogenous nature of melanoma (38). Rh-chymase decreased migration and proliferation of WM115 cells and at high concentration decreased to some extent the viability of G361 cells after detachment. Moreover, rh-chymase did not exhibit any stimulatory effect on melanoma cell lines. These findings support an antitumorigenic role of mast cells, proposed also by several in vivo studies (13-16), rather than a protumorigenic role. It is possible that the effects of mast cells and their chymase are not directly against melanoma cells, but come through effects on several other cell types present in the microenvironment. This is in line with previous findings in a mouse model, in which mast cells limited tumor growth and also controlled the recruitment of immune cells (39). Mast cells may be immunoregulatory by affecting complement C3-related pathology, as suggested in basal cell carcinoma [reviewed in (7)]. Recently, melanoma-secreted TGF-b and IL-1b were shown to upregulate C3 expression in melanoma-associated mast cells (40). Interestingly, chymase controls C3 effects by degrading C3, which has been reported in cutaneous vasculitis (27). The detailed mechanism of mast cell effects in melanoma seem to be complex and may depend on the state of melanoma pathogenesis. It is also possible that the role of mast cells changes during melanoma progression and is altogether very multifaceted.
Although chymase seems to be important in mast cell functions, the possible role of other mast cell proteinases or mediators should not be underestimated. Our data highlight the need of using many different melanoma cell lines in studies in vitro to see the variation, but also warrant future studies on the details of mast cell and melanoma interaction in different clinical settings to better understand the mechanism at the cellular level.
Acknowledgments
The authors thank Ms. Katja Dufva and Ms. Anne Koivisto for expert technical assistance.
Funding: This work was supported by research grants from
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1688/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1688/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1688/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1688/coif). HS reports receiving research funding from the Finnish Dermatological Society. ITH reports receiving funding from the Cancer Foundation Finland, the VTR-funding of Kuopio University Hospital and University of Eastern Finland. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Kuopio University Hospital and by the Finnish National Supervisory Authority for Welfare and Health (No. 71/2017). The registry study protocol was retrospective and thus the consent of the patients for participation was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siiskonen H, Smorodchenko A, Krause K, et al. Ultraviolet radiation and skin mast cells: Effects, mechanisms and relevance for skin diseases. Exp Dermatol 2018;27:3-8. [Crossref] [PubMed]
- Grimbaldeston MA, Simpson A, Finlay-Jones JJ, et al. The effect of ultraviolet radiation exposure on the prevalence of mast cells in human skin. Br J Dermatol 2003;148:300-6. [Crossref] [PubMed]
- Kim MS, Kim YK, Lee DH, et al. Acute exposure of human skin to ultraviolet or infrared radiation or heat stimuli increases mast cell numbers and tryptase expression in human skin in vivo. Br J Dermatol 2009;160:393-402. [Crossref] [PubMed]
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol 2013;31:4172-8. [Crossref] [PubMed]
- Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol 2011;65:S17-25.e1-3.
- Greinert R, de Vries E, Erdmann F, et al. European Code against Cancer 4th Edition: Ultraviolet radiation and cancer. Cancer Epidemiol 2015;39 Suppl 1:S75-83.
- Elieh Ali Komi D, Jalili A. The emerging role of mast cells in skin cancers: involved cellular and molecular mechanisms. Int J Dermatol 2022;61:792-803. [Crossref] [PubMed]
- Tóth-Jakatics R, Jimi S, Takebayashi S, et al. Cutaneous malignant melanoma: correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum Pathol 2000;31:955-60. [Crossref] [PubMed]
- Ribatti D, Ennas MG, Vacca A, et al. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest 2003;33:420-5. [Crossref] [PubMed]
- Ribatti D, Vacca A, Ria R, et al. Neovascularisation, expression of fibroblast growth factor-2, and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur J Cancer 2003;39:666-74. [Crossref] [PubMed]
- Grimbaldeston MA, Pearce AL, Robertson BO, et al. Association between melanoma and dermal mast cell prevalence in sun-unexposed skin. Br J Dermatol 2004;150:895-903. [Crossref] [PubMed]
- Molderings GJ, Zienkiewicz T, Homann J, et al. Risk of solid cancer in patients with mast cell activation syndrome: Results from Germany and USA. F1000Res 2017;6:1889. [Crossref] [PubMed]
- Biswas A, Richards JE, Massaro J, et al. Mast cells in cutaneous tumors: innocent bystander or maestro conductor? Int J Dermatol 2014;53:806-11. [Crossref] [PubMed]
- Dyduch G, Okoń K, Pescarini E. Mast cells in melanocytic skin lesions. An immunohistochemical and quantitative study. Pol J Pathol 2011;62:139-44. [PubMed]
- Siiskonen H, Poukka M, Bykachev A, et al. Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res 2015;25:479-85. [Crossref] [PubMed]
- Rajabi P, Bagheri A, Hani M. Intratumoral and Peritumoral Mast Cells in Malignant Melanoma: An Immunohistochemical Study. Adv Biomed Res 2017;6:39. [Crossref] [PubMed]
- Huttunen M, Harvima IT. Mast cell tryptase and chymase in chronic leg ulcers: chymase is potentially destructive to epithelium and is controlled by proteinase inhibitors. Br J Dermatol 2005;152:1149-60. [Crossref] [PubMed]
- Diaconu NC, Rummukainen J, Naukkarinen A, et al. Mast cell chymase is present in uterine cervical carcinoma and it detaches viable and growing cervical squamous carcinoma cells from substratum in vitro. Arch Dermatol Res 2011;303:499-512. [Crossref] [PubMed]
- Huttunen M, Hyttinen M, Nilsson G, et al. Inhibition of keratinocyte growth in cell culture and whole skin culture by mast cell mediators. Exp Dermatol 2001;10:184-92. [Crossref] [PubMed]
- Goldstein SM, Leong J, Schwartz LB, et al. Protease composition of exocytosed human skin mast cell protease-proteoglycan complexes. Tryptase resides in a complex distinct from chymase and carboxypeptidase. J Immunol 1992;148:2475-82. [PubMed]
- Weber A, Knop J, Maurer M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br J Dermatol 2003;148:224-8. [Crossref] [PubMed]
- Schwartz LB, Irani AM, Roller K, et al. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol 1987;138:2611-5. [PubMed]
- Vuoriluoto K, Jokinen J, Kallio K, et al. Syndecan-1 supports integrin alpha2beta1-mediated adhesion to collagen. Exp Cell Res 2008;314:3369-81. [Crossref] [PubMed]
- Kofford MW, Schwartz LB, Schechter NM, et al. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem 1997;272:7127-31. [Crossref] [PubMed]
- McEuen AR, Sharma B, Walls AF. Regulation of the activity of human chymase during storage and release from mast cells: the contributions of inorganic cations, pH, heparin and histamine. Biochim Biophys Acta 1995;1267:115-21. [Crossref] [PubMed]
- Walter M, Plotnick M, Schechter NM. Inhibition of human mast cell chymase by secretory leukocyte proteinase inhibitor: enhancement of the interaction by heparin. Arch Biochem Biophys 1996;327:81-8. [Crossref] [PubMed]
- Lipitsä T, Naukkarinen A, Laitala J, et al. Complement C3 is expressed by mast cells in cutaneous vasculitis and is degraded by chymase. Arch Dermatol Res 2016;308:575-84. [Crossref] [PubMed]
- Lipitsä T, Siiskonen H, Naukkarinen A, et al. Mast cell chymase degrades fibrinogen and fibrin. Br J Dermatol 2019;181:296-303. [Crossref] [PubMed]
- Leskinen MJ, Lindstedt KA, Wang Y, et al. Mast cell chymase induces smooth muscle cell apoptosis by a mechanism involving fibronectin degradation and disruption of focal adhesions. Arterioscler Thromb Vasc Biol 2003;23:238-43. [Crossref] [PubMed]
- den Dekker WK, Tempel D, Bot I, et al. Mast cells induce vascular smooth muscle cell apoptosis via a toll-like receptor 4 activation pathway. Arterioscler Thromb Vasc Biol 2012;32:1960-9. [Crossref] [PubMed]
- Ebihara N, Takai S, Miyazaki M, et al. Mast cell chymase induces conjunctival epithelial cell apoptosis by a mechanism involving degradation of fibronectin. Curr Eye Res 2005;30:429-35. [Crossref] [PubMed]
- Heikkilä HM, Lätti S, Leskinen MJ, et al. Activated mast cells induce endothelial cell apoptosis by a combined action of chymase and tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol 2008;28:309-14. [Crossref] [PubMed]
- Visciano C, Liotti F, Prevete N, et al. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene 2015;34:5175-86. [Crossref] [PubMed]
- Öhrvik H, Grujic M, Waern I, et al. Mast cells promote melanoma colonization of lungs. Oncotarget 2016;7:68990-9001. [Crossref] [PubMed]
- Groschwitz KR, Ahrens R, Osterfeld H, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A 2009;106:22381-6. [Crossref] [PubMed]
- Firth JD, Uitto VJ, Putnins EE. Mechanical induction of an epithelial cell chymase associated with wound edge migration. J Biol Chem 2008;283:34983-93. [Crossref] [PubMed]
- Huttunen M, Aalto ML, Harvima RJ, et al. Alterations in mast cells showing tryptase and chymase activity in epithelializating and chronic wounds. Exp Dermatol 2000;9:258-65. [Crossref] [PubMed]
- Shannan B, Perego M, Somasundaram R, et al. Heterogeneity in Melanoma. Cancer Treat Res 2016;167:1-15. [Crossref] [PubMed]
- Siebenhaar F, Metz M, Maurer M. Mast cells protect from skin tumor development and limit tumor growth during cutaneous de novo carcinogenesis in a Kit-dependent mouse model. Exp Dermatol 2014;23:159-64. [Crossref] [PubMed]
- Bahri R, Kiss O, Prise I, et al. Human Melanoma-Associated Mast Cells Display a Distinct Transcriptional Signature Characterized by an Upregulation of the Complement Component 3 That Correlates With Poor Prognosis. Front Immunol 2022;13:861545. [Crossref] [PubMed]




