
Inhibitory role of puerarin on the A549 lung cancer cell line
Introduction
Puerarin as a traditional Chinese medicine, was isolated in the late 1950s. Since then, its pharmacological properties were widely explored. Due to its wide spectrum of pharmacological properties, the beneficial effects of puerarin were quite widely, such as vasodilation, cardioprotection, neuroprotection, antioxidant, anticancer, antiinflammation, alleviating pain, promoting bone formation, inhibiting alcohol intake, and attenuating insulin resistance (1). However, the direct molecular mechanisms and targets were quite limited, especially in lung cancer.
Lung cancer is a common tumor that seriously threatens human health, with high rates of incidence and mortality. The majority of cases are in the advanced stage when they are first diagnosed. The unlimited proliferation of tumor cells is the most difficult obstacle in tumor therapy. After years of research, it has been established that tumor cells escape radiotherapy and drug therapy through a variety of mechanisms, among which is the invasion of tumor tissue and metastasis to surrounding normal tissue (2). At present, the identification of potential drugs that can effectively block the invasion of tumor cells into surrounding tissues at the known important nodes of tumor cell survival is a focus of drug research and development. This approach aims to limit tumors to a fixed position for easy removal.
Tumor metastasis is a complex, multi-step, and multi-gene-regulated process, and is the main cause of death (3). The process of invasion and metastasis of malignant tumor cells is accompanied by the degradation of the extracellular matrix, and matrix metalloproteinases (MMPs) are the most important hydrolases involved in this process (4,5). Gelatinase is an important subtype of the MMP family, including MMP-9. It can not only degrade the extracellular matrix components, but also degrades type IV collagen, which is the main component of the basement membrane, destroys the integrity of the basement membrane, and provides a basis for tumor cell invasion and metastasis (6,7). In addition, previous reports have shown that the increased expression of MMP9 in lung cancer is associated with poor prognosis (8-10). Therefore, the invasion and metastasis of melanoma cells can be inhibited by blocking the effects of MMP9.
The process of tumor invasion and metastasis involves the abnormalities of multiple cellular signal transduction pathways. The mitogen-activated protein kinase (MAPK) signaling pathway is an important signal transduction system for eukaryotic cells to mediate extracellular signals to intracellular reactions. It is activated by phosphorylation after stimulation and regulates the processes of cell growth, differentiation, division, death, and functional synchronization between cells (11-13), including extracellular signal-regulated kinase, c-Jun N-terminal kinase, p38 mitogen-activated protein kinase, and extracellular-regulated kinase 5. The ERK pathway is a classic pathway of MAPK, which is involved in the regulation of cell proliferation, tumor invasion, and metastasis.
Lung cancer exhibits characteristics such as strong invasion, easy recurrence and metastasis, and poor prognosis. At present, the commonly used clinical chemotherapy regimen has strong toxic and side effects, and thus, there is a pressing need to identify suitable adjuvant drugs that reduce these effects. Kudzu vine is a widely used traditional Chinese medicine in the clinic. Puerarin is the main extract of kudzu vine, and a previous study has shown that it has a significant effect on tumor necrosis factor, interferon, and other cytokines (14). At present, puerarin is mainly used to treat cardiovascular diseases; however, a summary of the previous research literature highlighted numerous pathways involved in its treatment that overlap with the pathways through which tumor metastasis develops. Therefore, this study aims to investigate the effect of puerarin on the growth and metastasis of A549 lung cancer cells in vivo and in vitro and preliminarily discuss its mechanism to assist in the clinical treatment of lung cancer. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2246/rc).
Methods
Reagents and equipment
A 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) kit was purchased from Promega (USA). MMP9, ERK1/2, and phosphorylated ERK1/2 (p ERK1/2) antibodies were purchased from Abcam (USA). An RNA extraction kit, reverse transcription kit, and quantitative polymerase chain reaction (qPCR) SuperMIX were purchased from TAKARA (Japan). Puerarin was purchased from Sigma (USA). Matrigel was purchased from BD (USA). The gene amplification instrument and fluorescence imaging system were obtained from Bio-RAD (USA). A microscopic imaging system (Leica, Germany) from Germany was also used.
Cell survival rate
The A549 human lung cancer cell line (ATCC CCL-185, USA) was cultured with Roswell Park Memorial Institute (RPMI) 1640 (GIBCO, USA) with 10% fatal bovine serum (FBS) (GIBCO, USA) in 37 ℃ and 5% carbon dioxide (CO2). A medium with different concentrations of puerarin (10, 20, 40, 80, and 160 µmol/L was added to a 96-well plate, and the A549 cells were adjusted to 5×103/well. The cell survival rate was detected with MTT [optical density (OD) =450 nm] after 24 hours.
Cell scratch
The experimental grouping and dosage were determined using the cell survival rate experiment, and doses of 10, 20, and 40 µmol/L were adopted. The A549 cells were adjusted to 1×105/35 mm tissue culture dish. Each group had three dishes. The cells were cultured until they completely adhered to the wall, the center of the culture dish was scratched with the tip, and the cells were then immediately washed with phosphate buffered solution (PBS). Next, the culture medium containing different concentrations of puerarin was added, photos were taken under the microscope, and the cells were then allowed to continue culturing in the standard culture environment for 24 hours. Subsequently, photos were taken again to calculate the scratch healing rate.
Transwell
The Matrigel was melted at 4 ℃ and 100 µL was added into each Transwell chamber, which was shaken gently to cover the bottom of the chamber evenly and maintained at 37 ℃ for 30 min to fully solidify. The cells were adjusted with the medium to 1×104 cell/chamber. Next, the different concentrations of puerarin (10, 20, and 40 µmol/L) were added to each chamber and a control group was set at the same time, with three multiple holes in each group. The chambers were placed on the 24-well plate with the same medium correspondingly, and the bottom of the chamber was immersed in the medium. After culturing in a standard cell culture environment for 24 hours, the chamber was taken out and washed with PBS. The bottom of the chamber was fixed using absolute ethanol for 30 min. After drying, the bottom of the cell was stained with crystal violet staining solution for 10 min. After cleaning and drying, we observed and took photos under the microscope, counted, and then compared the number of invasive cells in each group.
Gene expression analysis
The experimental grouping and dosages were the same as those mentioned above, as determined by the cell survival rate experiment. Total RNA was extracted after 24 hours in the puerarin-containing medium and reverse-transcribed into complementary DNA (cDNA) for fluorescence quantitative PCR. The reaction conditions were pre-denaturation at 95 ℃ for 10 min, amplification cycle conditions at 95 ℃ for 10 s, and 60 ℃ for 60 s, for a total of 40 cycles. The primer sequences were as follows: (5'-3'), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference. The MMP9 primer was as follows: (F) GGGACGCAGACATCGTCATC; (R) TCGTCATCGTCGAAATGGGC.
Western blotting
The experimental grouping and dosages were the same as those mentioned above, as determined by the cell survival rate experiment. Protein was extracted from cells with protein lysate, and the protein concentration was determined using a bicinchoninic acid assay (BCA) kit. Next, the protein was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride (PVDF) membrane, blocked with tris buffered saline (TBS) containing 5% bovine serum albumin (BSA) and 0.1% Tween-20, and incubated with primary antibodies overnight at 4 ℃. The membranes were then incubated with horseradish peroxidase-labeled secondary antibody, and enhanced chemiluminescence (ECL) was finally developed.
Statistical analysis
Data were presented as the means ± standard error of the mean (SEM). Normally distributed data were compared using the unpaired Student’s t-test for two groups comparisons. P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). All biological replicates were performed at least three times. The experiment and analysis were double-blinded.
Results
Cytotoxic effect of puerarin on A549 cells
The MTT results were used to select the experimental concentration of puerarin. After treatment with puerarin for 24 hours, high concentrations of puerarin had an effect on the survival rate of A549 cells, and the inhibitory effect was concentration-dependent. According to the P value, >80 µmol/L had a significant inhibitory effect. To reduce the influence of the drug’s cytotoxic effect on migration and invasion, puerarin concentrations of 10, 20, and 40 µmol/L were used in the subsequent experiments (Figure 1).
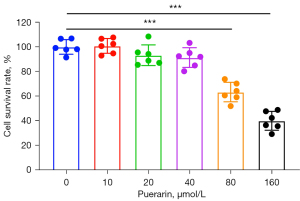
Effects of puerarin on the invasion and metastasis of A549 cells
According to the drug toxicity results, three concentrations (10, 20, and 40 µmol/L) were selected for detection. The cell invasion results showed that compared with the control group, the scratch healing rate of the puerarin-treated groups decreased significantly after 24 h, suggesting that puerarin can inhibit tumor metastasis by inhibiting the migration ability of A549 cells. The cell metastasis results demonstrated that the level of cell invasion in each puerarin treatment group was significantly lower than that in the control group, indicating that puerarin can inhibit cancer metastasis by inhibiting metastatic ability. The experimental results were concentration-dependent (Figure 2).
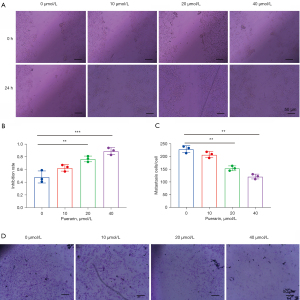
Puerarin affects cell migration and metastasis-related factors to prevent tumor metastasis
Total RNA and protein were extracted 24 h after puerarin administration at 10, 20, and 40 µmol/L. The gene and protein levels of the proliferation-related factor, MMP9, were detected. The qPCR results showed that compared with the control group, the expression level of MMP9 decreased significantly after puerarin treatment. With the increasing dose, the range of change increased in a dose-dependent manner. The difference between the groups was statistically significant. As shown in Figure 3A, puerarin can inhibit or activate the messenger RNA (mRNA) expression level of MMP9.
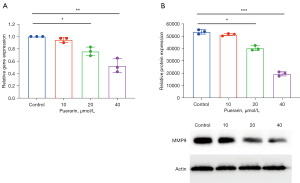
Western blotting further illustrated that the MMP9 expression levels in A549 cells of the control group were high, while puerarin could significantly inhibit this expression (Figure 3B). Moreover, the protein expression difference was also statistically significant, which is consistent with the gene expression level. These results suggest that puerarin markedly inhibits the metastasis of tumor cells.
Puerarin prevents tumor metastasis by inhibiting the phosphorylation of ERK pathways
The upstream pathways ERK were used to further confirm the mechanism of puerarin on tumor metastasis-related factors. The western blot results (Figure 4A,4B) showed that the expression of p-ERK in A549 cells was inhibited after puerarin treatment, indicating that ERK pathway activity had decreased. In addition, the protein expression results highlighted that the difference between the groups was statistically significant.
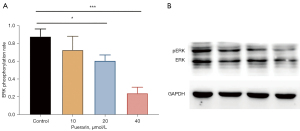
Discussion
Invasion and metastasis are important characteristics of malignant tumors and are also the main causes of treatment failure and mortality in tumor patients. Considering this, the development of new treatment strategies that hinder metastasis is expected to improve the treatment of tumors (15). Tumor metastasis involves a series of highly related biological processes, such as the adhesion changes between cells or extracellular matrix, cell migration, and cell invasion to the basement membrane and extracellular matrix.
The MMP family is closely related to the invasion and metastasis of lung cancer. As an important component of MMPs, MMP9 plays an important role in the invasion and metastasis of tumor tissues and cells. In this study, the Transwell invasion experiment results showed that puerarin could significantly reduce the number of invasive cells. Since MMPs possess extracellular matrix degradation and tissue remodeling abilities, numerous stages of tumor progression, such as tumor formation, growth, angiogenesis, and metastasis, are related to the activity of MMPs (16). In particular, the enhanced expression of MMP9 plays an important role in tumor cell invasion. Several studies have found that MMP9 levels in lung cancer tissues are significantly higher compared to that in adjacent normal tissues, and are positively correlated with lymph node metastasis and clinical stage (17-19). Therefore, the invasion of tumor cells can be limited by inhibiting MMP9 or regulating signal pathways, so as to inhibit metastasis (20-22). Puerarin can significantly reduce the protein expression levels of MMP9 in A549 cells, suggesting that puerarin can inhibit cell invasion by reducing the expression of MMP9.
Following in-depth research, the MAPK signaling pathway has been established as the key pathway regulating the expression of MMP9. This pathway is an important signal transduction system that mediates extracellular stimulation to the intracellular response. It also regulates cell proliferation, differentiation, and apoptosis; participates in the regulation of cell growth and development; and plays an important role in the occurrence and infiltration of malignant tumor cells (23). Numerous studies have shown that the MAPK pathway in the signal pathway plays an important role in the process of ERK signal transmission, and it is also the most closely related pathway to human cancer (24-26).
P-ERK is the activated form of ERK, and only phosphorylated ERK exerts activity. ERK can promote the transcription and expression of many oncogene-related genes through phosphorylation, destroy extracellular matrix, and promote tumor angiogenesis, which is conducive to the movement of tumor cells and metastasis. The ERK signaling pathway is typically over-activated in several tumors (27,28). Also, studies have confirmed that this pathway plays an important role in regulating the proliferation, survival, and invasiveness of lung cancer cells (29-31). Our results showed that puerarin could inhibit ERK signaling. In this study, the western blotting and qPCR results showed that puerarin had a significant effect on the expression of MMP in A549 cells; the expression of MMP9 was negatively correlated with the dose. These changes were beneficial to inhibit the invasion and metastasis of lung cancer.
In 2016, Chen et al. first reported puerarin 6"-O-xyloside role in antitumour activities via the induction of the mitochondria-mediated apoptosis pathway (32), then another several studies reported different mechanisms of puerarin, including alleviates the progression by regulating the miR-342/CCND1 axis (33); suppresses growth, self-renewal and invasion via regulating Akt/c-Myc signalling (34); inhibits the proliferation, invasion, and migration through regulating miR-490/Denticleless E3 Ubiquitin Protein Ligase (35); inhibits M2 polarization and metastasis of tumor-associated macrophages from NSCLC xenograft model via inactivating MEK/ERK 1/2 pathway (36). Up to now, no research focus on the MMP9 and ERK pathways.
In conclusion, puerarin is an anti-tumor drug with broad prospects. It exerts an obvious inhibitory effect on tumor metastasis. Further research and development will be carried out in future research to provide a technical basis for the development of potential lung cancer treatment drug candidates with low toxicity and high activity.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2246/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2246/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2246/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res 2014;28:961-75. [Crossref] [PubMed]
- Romaszko AM, Doboszynska A. Multiple primary lung cancer: A literature review. Adv Clin Exp Med 2018;27:725-30. [Crossref] [PubMed]
- Babaei G, Aziz SG, Jaghi NZZ. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed Pharmacother 2021;133:110909. [Crossref] [PubMed]
- Huang H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors (Basel) 2018;18:3249. [Crossref] [PubMed]
- Wu HT, Lin J, Liu YE, et al. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021;81:153437. [Crossref] [PubMed]
- Jiang K, Chen H, Fang Y, et al. Exosomal ANGPTL1 attenuates colorectal cancer liver metastasis by regulating Kupffer cell secretion pattern and impeding MMP9 induced vascular leakiness. J Exp Clin Cancer Res 2021;40:21. [Crossref] [PubMed]
- Kwon Y, Park SJ, Nguyen BT, et al. Multi-layered proteogenomic analysis unravels cancer metastasis directed by MMP-2 and focal adhesion kinase signaling. Sci Rep 2021;11:17130. [Crossref] [PubMed]
- Green D, Eyre H, Singh A. Targeting the MAPK7/MMP9 axis for metastasis in primary bone cancer. 2020;39:5553-69.
- Gu JJ, Hoj J, Rouse C, et al. Mesenchymal stem cells promote metastasis through activation of an ABL-MMP9 signaling axis in lung cancer cells. 2020;15:e0241423.
- Wu D, Deng S, Li L, et al. TGF-β1-mediated exosomal lnc-MMP2-2 increases blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death Dis 2021;12:721. [Crossref] [PubMed]
- Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med 2006;38:200-11. [Crossref] [PubMed]
- Tian W, Yang X, Yang H, et al. Exosomal miR-338-3p suppresses non-small-cell lung cancer cells metastasis by inhibiting CHL1 through the MAPK signaling pathway. 2021;12:1030.
- Yan P, Zhu H, Yin L, et al. Integrin αvβ6 Promotes Lung Cancer Proliferation and Metastasis through Upregulation of IL-8-Mediated MAPK/ERK Signaling. Transl Oncol 2018;11:619-27. [Crossref] [PubMed]
- Zeng YP, Yang ZR, Guo XF, et al. Synergistic effect of puerarin and 5-fluorouracil on hepatocellular carcinoma. Oncol Lett 2014;8:2436-42. [Crossref] [PubMed]
- Zeng R, Huang JP, Li XF, et al. Epb41l3 suppresses esophageal squamous cell carcinoma invasion and inhibits MMP2 and MMP9 expression. Cell Biochem Funct 2016;34:133-41. [Crossref] [PubMed]
- Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol 2012;181:1895-9. [Crossref] [PubMed]
- Zeng F, Yu N, Han Y, et al. The long non-coding RNA MIAT/miR-139-5p/MMP2 axis regulates cell migration and invasion in non-small-cell lung cancer. J Biosci 2020;45:51. [Crossref] [PubMed]
- Li H, Huang N, Zhu W, et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018;18:579. [Crossref] [PubMed]
- Zeng Y, Wang X, Yin B, et al. Role of the stromal cell derived factor-1/CXC chemokine receptor 4 axis in the invasion and metastasis of lung cancer and mechanism. J Thorac Dis 2017;9:4947-59. [Crossref] [PubMed]
- Wang L, Wang Q, Li HL, et al. Expression of MiR200a, miR93, metastasis-related gene RECK and MMP2/MMP9 in human cervical carcinoma--relationship with prognosis. Asian Pac J Cancer Prev 2013;14:2113-8. [Crossref] [PubMed]
- Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis 2005;22:237-46. [Crossref] [PubMed]
- Wang X, Yang B, She Y, et al. The lncRNA TP73-AS1 promotes ovarian cancer cell proliferation and metastasis via modulation of MMP2 and MMP9. 2018;119:7790-9.
- Hsiao YC, Kuo WH, Chen PN, et al. Flavanone and 2'-OH flavanone inhibit metastasis of lung cancer cells via down-regulation of proteinases activities and MAPK pathway. Chem Biol Interact 2007;167:193-206. [Crossref] [PubMed]
- Chao W, Deng JS, Li PY, et al. 3,4-Dihydroxybenzalactone Suppresses Human Non-Small Cell Lung Carcinoma Cells Metastasis via Suppression of Epithelial to Mesenchymal Transition, ROS-Mediated PI3K/AKT/MAPK/MMP and NFκB Signaling Pathways. Molecules 2017;22:537. [Crossref] [PubMed]
- Yu T, Bai W, Su Y, et al. Enhanced expression of lncRNA ZXF1 promotes cisplatin resistance in lung cancer cell via MAPK axis. Exp Mol Pathol 2020;116:104484. [Crossref] [PubMed]
- Wu Z, He D, Zhao S, et al. IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer. Mol Cell Biochem 2019;455:195-206. [Crossref] [PubMed]
- Zhang X, Zhang Y, Miao Y, et al. TMEM17 depresses invasion and metastasis in lung cancer cells via ERK signaling pathway. Oncotarget 2017;8:70685-94. [Crossref] [PubMed]
- Zhang X, He Y, Jiang Y, et al. TMEM229A suppresses non-small cell lung cancer progression via inactivating the ERK pathway. Oncol Rep 2021;46:176. [Crossref] [PubMed]
- Lou Z, Lin W, Zhao H, et al. Alkaline phosphatase downregulation promotes lung adenocarcinoma metastasis via the c-Myc/RhoA axis. Cancer Cell Int 2021;21:217. [Crossref] [PubMed]
- Zhang B, Qiao T, Gao C. Effects and mechanism of ensartinib (X-396) on the adhesion and metastasis of non-small cell lung cancer cells. Pharmazie 2019;74:543-6. [PubMed]
- Liu C, Li H, Jia J, et al. High Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1) Expression Promotes Proliferation, Migration, and Invasion of Non-Small Cell Lung Cancer via ERK/Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway. Med Sci Monit 2019;25:5143-9. [Crossref] [PubMed]
- Chen T, Chen H, Wang Y, et al. In vitro and in vivo antitumour activities of puerarin 6''-O-xyloside on human lung carcinoma A549 cell line via the induction of the mitochondria-mediated apoptosis pathway. Pharm Biol 2016;54:1793-9. [Crossref] [PubMed]
- Huang SR, Jin SS, Xu B, et al. Puerarin alleviates the progression of non-small cell lung cancer by regulating the miR-342/CCND1 axis. Neoplasma 2020;67:1244-55. [Crossref] [PubMed]
- Tao X, Yin Y, Lian D, et al. Puerarin 6''-O-xyloside suppresses growth, self-renewal and invasion of lung cancer stem-like cells derived from A549 cells via regulating Akt/c-Myc signalling. Clin Exp Pharmacol Physiol 2020;47:1311-9. [Crossref] [PubMed]
- Zhang YX, Zhang ZZ, Zhao LG, et al. Puerarin Inhibits the Proliferation,Invasion,and Migration of Non-small Cell Lung Cancer Cells through Regulating miR-490/Denticleless E3 Ubiquitin Protein Ligase. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2022;44:91-101. [PubMed]
- Kang H, Zhang J, Wang B, et al. Puerarin inhibits M2 polarization and metastasis of tumor-associated macrophages from NSCLC xenograft model via inactivating MEK/ERK 1/2 pathway. Int J Oncol 2017;50:545-54. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


