
Mixed primary mucinous cystadenocarcinoma and invasive ductal carcinoma of the breast: a case report and literature review
Introduction
In 1998, Koenig and Tavassoli (1) were first to describe the primary mucinous cystadenocarcinoma (MCA) of the breast. This breast disease usually presents clinically as unilateral, well-circumscribed cystic and/or solid masses, and it occurs predominantly in postmenopausal women but with a favorable outcome. In the 2002 World Health Organization (WHO) classification of Pathology and Genetics of Breast and Female Reproductive Organs (2), MCA, mucinous carcinoma (MC), signet ring cell carcinoma, and columnar cell MC, were classified as mucin-producing carcinomas. However, the classification of MCA was removed from the 2012 WHO classification, possibly due to the low incidence of MCA. In the 2019 WHO classification of breast (3), MCA was listed separately in the invasive breast carcinoma category. The MCA is a less aggressive neoplasm with good prognosis. Only five cases of the breast MCA mixed with invasive ductal carcinoma (IDC) have been reported, and its pathogenesis is under investigation. We present a 61-year-old woman diagnosed as mixed primary MCA and IDC of the breast though histological examination and immunohistochemical analysis, combined with detailed clinical and imaging information. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1596/rc).
Case presentation
A 61-year-old postmenopausal woman who had no family history of breast cancer was admitted to our hospital due to a palpable mass in her left breast lasting for 2 months. In the examination, the surgeon touched a 25×20 mm mass with firm texture in the upper outer quadrant of the breast. No palpable axillary lymph node was found. Neither skin retraction nor nipple discharge was seen. Ultrasonography demonstrated a 2 to 3 o’clock cystic-solid lump with clear boundary and irregular contour at the left breast (Figure 1A). Breast Imaging-Reporting and Data System (BI-RADS) classification was 4b. Magnetic resonance imaging (MRI) showed an irregular lobulated mass of 21×19×16 mm, 38 mm from the nipple. The mass showed low signal intensity on T1 weighted imaging and high signal intensity on T2 weighted imaging, along with rim irregularly-enhancement and spiculate boundary. Core needle biopsy of this lesion showed most areas were intermediate-grade IDC and ductal carcinoma in situ (DCIS). Twenty percent of the lesions showed detached papillary structure or fragments of epithelial tissue lined by tall columnar cells (Figure 1B). Intraoperative frozen sections showed metastatic IDC cells in one of four sentinel lymph nodes (Figure 1C). Therefore, the patient underwent left modified radical mastectomy with axillary dissection.
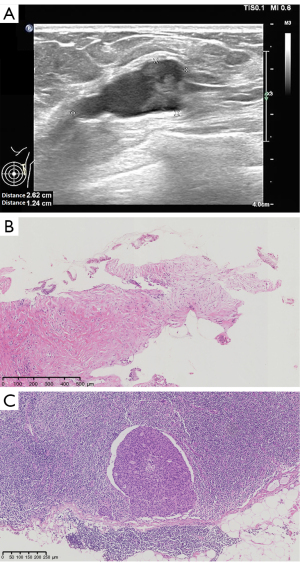
Overall, the tumor presented as a multilocular cyst filled with white gelatinous material (maximum diameter about 21 mm; Figure 2). The nipple and the rest of the breast tissue did not show any abnormality. Microscopically, most of the tumor was cystic structure with papillary projections, and a few were solid areas (Figure 3A). The cystic spaces and the papillary structures with multiple delicate fibrovascular stalks were both covered by a monomorphic population of tall columnar neoplastic epithelial cells containing rich intracytoplasmic mucin and basally located nuclei (Figure 3B,3C). Clusters of neoplastic cells were also seen within pools of extracellular mucin, similar to MC (Figure 3D). In some solid areas, foci of IDC (diameter about 8 mm) and intermediate-high grade DCIS were observed around the MCA (Figure 3E). At a high-power microscopic view of the MCA, the crowded epithelial cells with intermediate-grade nuclei and inconspicuous nucleolus were arranged in single or multiple cell layers (Figure 3F). The number of mitotic figures of this tumor was 12 per 10 high-power fields. The Nottingham Grading System score of MCA and IDC were both grade 2. There was no transitional zone between MCA and IDC or DCIS. No metastatic tumor cells were found in the axillary lymph nodes (0/15). Lymphovascular or perineural invasion was not present.


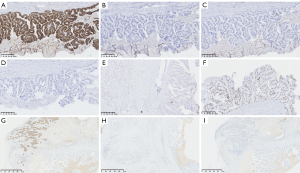
Immunohistochemistry of MCA (Figure 4A-4I), ER, PR, HER2, CK20, AR, CK5/6, MUC2, and CDX2 were negative. CK7, MUC1, and MUC5AC were positive. Myoepithelial cells were not present around the tumor cell nests with a lack of staining for P63 except for DCIS. Immunohistochemistry of IDC (Figure 4G-4I) showed expression of ER (90%+), PR (−), HER2 (−), and AR (50%+). The Ki67 proliferation index of MCA and IDC were both 40%. Next generation sequencing did not detect the point mutation or insertion-deletion mutations of whole coding region, exon-intron junction region, UTR region (untranslated region), and promoter region of BRCA1 (NM_007294.3; mutation analysis for exons 2, 3, 5~24) and BRCA2 (NM000059.3; mutation analysis for exons 2~27) in our case.
The final diagnosis was mixed primary MCA (60%) and IDC of the breast. According to the Eighth American Joint Committee on Cancer Staging System, the tumor was staged as pT2N1M0. After surgery, the patient underwent eight cycles of chemotherapy consisting of pharmorubicin, cyclophosphamide, docetaxel, and paclitaxel. She responded well, and she did not have any recurrence, distant metastasis, or tumor-related disease during the 10 months follow-up, although accompanied by sentinel lymph node metastasis and a triple-negative immunophenotype. The timeline of diagnosis and therapy is shown in Figure 5.
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We searched the PubMed database. Up to 2021, there were 31 reported cases of primary MCA in the breast, of which only 5 were mixed MCA and IDC of the breast. Table 1 and Table 2 summarize the clinical and pathological characteristics of each patient, respectively. Patients were aged from 41 to 96 years, with an average age of 62 years, about 6 to 7 years younger than patients with MC (29). Fifty-six percent of the patients (18/32) were over 60, including the patient reported here. Two of them were aged 41 (4) and 45 (5), which suggested that MCA can also occur in premenopausal women. Of the 32 patients, four had a history of contralateral or ipsilateral breast tumor (6-9). The remaining patients did not have family history of breast cancer or other breast-related diseases. The size of MCA at diagnosis was described from non-palpable lesions to 190 mm (1,10,11), and the average size was 44 mm, larger than the tumor in this case study.
Table 1
| Case/reference | Age (years) | Family history/medical history | Side | Size (mm) | Clinical manifestation | pTNM (AJCC) | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 (1) | 54 | No | Right | 190 | Large mass with skin ulceration | T4bN1M0 | M, LND (2/23) | ANED, 24 months |
| 2 (1) | 67 | No | Right | 23 | Palpable mass | T2N0M0 | M, LND (0/12) | ANED, 22 months |
| 3 (1) | 49 | No | Left | 85 | Palpable mass | T3N0M0 | M, LND (0/19), C, R | ANED, 11 months |
| 4 (1) | 61 | No | Left | 8 | Mammographic mass | T1bN0M0 | Lu, LND (0/3) | NA |
| 5 (4) | 41 | No | Left | 70/50/25 | Palpable mass | T3N1M0 | M, LND (3/14) | ANED, 24 months |
| 6 (5) | 45 | No | Left | 43 | Palpable mass | T2N0M0 | M, LND, C, R | ANED, 6 months |
| 7 (6) | 63 | Lu, LND, C, R, in CB | Left | 16 | Palpable mass | T1cN1M0 | Lu, LND (2/13), C, R | ANED, 48 months |
| 8 (7) | 58 | M, LND, C, R, in CB | Left | 45 | Palpable mass | T2N0M0 | M, LND | ANED, 6 months |
| 9 (8) | 68 | No | Left | 62 | Palpable mass | T3N0M0 | Lu, SLND | ANED, 3 months |
| 10 (8) | 59 | Lu, LND, SLND (itc+) in IB | Left | 20 | Palpable mass | T1cN0 (i+) M0 | Lu | Alive with local recurrence after 96 months |
| 11 (9) | 52 | Lu in IB | Right | 65 | Palpable mass | T3N0M0 | M, LND, C | ANED, 12 months |
| 12 (10) | 59 | No | Left | 9 | Palpable mass | T1bN0M0 | PM, SLND, C | ANED, 3 months |
| 13 (11) | 72 | No | Right | 9 | Mammographic mass | T1bN0M0 | PM, SLND, R | ANED, 16 months |
| 14 (12) | 79 | NA | NA | 60 | Palpable mass, skin retraction, nipple deformity | T3N0M0 | M | 9 years DOR |
| 15 (13) | 91 | No | Left | 75 | Palpable mass, nipple inversion | T3N0M0 | M, LND (0/15), R | 14 months DOR |
| 16 (14) | 69 | No | Left | 20 | Palpable mass, nipple discharge | T1cN0M0 | M, LND (0/16), HT | NA |
| 17 (15) | 96 | No | Left | 20 | Palpable mass | T1cN1M0 | Lu, LND | 46 months DOR |
| 18 (16) | 65 | No | Right | 30 | Palpable mass | T2N0M0 | M, LND, C | ANED, 8 months |
| 19 (17) | 55 | ATH with BSO | Right | 25 | Palpable mass | T2N0M0 | M, LND (0/12) | ANED, 6 months |
| 20 (18) | 65 | No | Right | 30 | Palpable mass | T2N0M0 | Lu, LND | ANED, 6 months |
| 21 (19) | 52 | No | Left | 100 | Palpable mass | T3N0M0 | M, LND (0/10), TMX | ANED, 24 months |
| 22 (20) | 73 | No | Left | 45 | Palpable mass | T2N0M0 | M, LND (0/12) | NA |
| 23 (21) | 55 | No | Left | 20 | Palpable mass | T1cN0M0 | M, SLND (0/3), C, R, TTZ | ANED, 10 months |
| 24 (22) | 59 | No | Left | 20 | Screening mammography mass | T1c | PM | NA |
| 25 (22) | 50 | No | Left | 22 | Palpable mass | T2 | PM | NA |
| 26 (23) | 74 | No | NA | 100 | Palpable mass | T3N0M0 | M, LND | ANED, 2 years |
| 27 (24) | 51 | No | Right | 40 | Palpable mass | T2N0M0 | Lu | NA |
| 28 (25) | 61 | No | Left | 30 | Subareolar cystic lump | T2N0M0 | M, LND | ANED, 6 months |
| 29 (26) | 62 | No | Right | 56 | Palpable mass | T3N0M0 | M, LND | ANED, 5 months |
| 30 (27) | 68 | D, AH | Right | 40 | Palpable mass | T2N0M0 | M, LND (0/15) | ANED, 21 months |
| 31 (28) | 66 | No | Right | 25 | Palpable mass | T2N0M0 | M, LND (0/17) | ANED, 13 months |
| Our case | 61 | No | Left | 21 | Palpable mass | T2N1M0 | M, SLND (1/4), LND (0/19), C | ANED, 10 months |
MCA, mucinous cystadenocarcinoma; TNM, T-primary tumor, N-regional lymph nodes, M-distant metastasis; AJCC, American Joint Committee on Cancer; M, modified radical mastectomy; LND, lymph node dissection; ANED, alive with no evidence of disease; C, chemotherapy; R, radiotherapy; Lu, lumpectomy; NA, not available; CB, contralateral breast; SLND, sentinel lymph node dissection; IB, ipsilateral breast; PM, partial mastectomy; DOR, died of other reason; HT, hormonal therapy; ATH with BSO, abdominal total hysterectomy for myoma uteri along with bilateral salpingo-oophorectomy; TMX, tamoxifen; TTZ, trastuzumab; itc, isolated tumor cell clusters; D, AH, diabetes, arterial hypertension.
Table 2
| Case/reference | Gross appearance | IDC | DCIS | ER/PR/HER2 | Ki67 | CK7/CK20 | MUC1/MUC5AC/MU6 |
|---|---|---|---|---|---|---|---|
| 1 (1) | Solid and cystic | − | − | −/−/NA | 40% | +/− | NA |
| 2 (1) | Cystic | − | + | −/−/NA | 30% | +/− | NA |
| 3 (1) | Solid and cystic | − | + | −/−/NA | 70% | +/− | NA |
| 4 (1) | Solid, gray-brown | − | − | −/−/NA | 50% | +/− | NA |
| 5 (4) | Well-defined cystic | − | + | −/−/− | 50% | +/− | NA |
| 6 (5) | Solid and cystic | − | + | −/−/− | 50% | +/+ | +/+/− |
| 7 (6) | Solid and cystic | − | − | −/−/− | NA | +/− | NA |
| 8 (7) | Solid and cystic | + | + | −/−/− | 80% | +/− | NA |
| 9 (8) | Cystic | − | + | −/−/− | NA | +/− | NA |
| 10 (8) | Cystic | − | + | −/−/− | NA | +/− | NA |
| 11 (9) | Multilocular cystic | − | − | −/−/− | 10% | +/− | NA |
| 12 (10) | Irregular, solid and firm | − | + | −/−/2+ | 5% | +/− | +/+/− |
| 13 (11) | Solid and cystic | − | − | −/−/− | 30% | +/− | NA |
| 14 (12) | Solid and cystic | + | + | NA | NA | NA | NA |
| 15 (13) | Solid and cystic | + | + | −/−/− | 40% | +/− | NA |
| 16 (14) | Solid and cystic | − | + | +/+/− | NA | NA | NA |
| 17 (15) | Multilocular cystic | − | − | −/−/− | 35% | +/− | NA |
| 18 (16) | Cystic | + | + | −/−/− | 20.5% | +/focally+ | NA |
| 19 (17) | Unilocular cystic | + | + | −/−/− | >90% | +/− | NA |
| 20 (18) | Unilocular cyst | − | + | −/−/− | NA | +/− | NA |
| 21 (19) | Well-defined multilobular cystic | − | − | +95%/−/− | NA | −/− | NA |
| 22 (20) | Lobulated cystic | − | + | −/−/2+ | NA | +/− | NA |
| 23 (21) | Solid and cystic | − | + | −/−/2+ | 30% | +/− | NA |
| 24 (22) | Multilocular cystic | − | − | −/−/3+ | NA | NA | NA |
| 25 (22) | Solid and cystic | − | − | −/−/− | NA | NA | NA |
| 26 (23) | Multilocular cystic | − | − | NA/NA/NA | 21.8% | +/− | NA |
| 27 (24) | Multilocular cystic | − | − | −/−/NA | NA | +/− | NA |
| 28 (25) | Unilocular cystic | − | − | −/−/− | NA | +/− | NA |
| 29 (26) | Solid and cystic | − | − | −/−/− | NA | +/− | NA |
| 30 (27) | Solid and cystic | − | − | −/−/− | NA | +/− | NA |
| 31 (28) | Solid and cystic | − | + | −/−/− | 60% | +/− | NA |
| Our case | Well-defined multilobular cystic | + | + | −/−/− | 40% | +/− | +/focally+/− |
+, positive; −, negative. MCA, mucinous cystadenocarcinoma; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ER, estrogen receptor; PR, progesterone receptor; NA, not available.
The clinical presentation of MCA was a palpable mass with occasional skin ulcers, skin retraction, or nipple discharge (1,12-14). For the previously reported cases, the lump of MCA appeared under the nipple and the areola of the breast; thus, some authors (15,16) considered that MCA might originate from the large lactiferous ducts of the nipple. However, for our case, MCA was about 38 mm from the nipple; thus, the location of MCA was not closely related to the nipple. DCIS was found adjacent to MCA in 18 cases, coexisting with IDC in five (7,12,13,16,17). In our case, MCA, IDC, and DCIS are all present. MCA was most frequently of a cystic nature with plentiful intra- and extra-cytoplasmic mucin. The neoplastic epithelial cells with bottom-placed nuclei formed a variety of structures, such as papillary, micropapillary, and tufting patterns. The cytological atypia can be either low or high grade. Other histological findings, such as squamous differentiation and sarcomatous component mixed with mucinous component can be present in the MCA (1,8,15,18).
Our MCA had a triple-negative phenotype, which was consistent with the majority of MCAs. However, two previous cases had ER expression (14,19) and received hormone therapy with tamoxifen, four had HER2 expression (10,20-22), and one of them chose trastuzumab therapy (21). One case had basal-like immunophenotype that was positive for CK5/6 and EGFR (4). Two were positive for CK20 and CK7 (5,16); one was negative for both CK20 and CK7 (19). The Ki67 proliferation index varied from 5% to over 90%. And there were 14 patients had Ki67 index higher or equal to 30%, including our case.
Only four cases of MCA had ipsilateral axillary lymph node metastasis. For mixed MCA and IDC cases, we were the first to report sentinel lymph node metastasis. Interestingly, the metastatic tumor cells were IDC cells instead of MCA cells, which suggested that the prognosis may be worse than that for pure MCA metastasis. Most patients, including the patient reported here, underwent radical mastectomy and ipsilateral axillary lymph node dissection and/or sentinel lymph node dissection. Ten received additional radiotherapy and/or chemotherapy. The duration of follow-up was from 3 months to 9 years. Only one had long-term focal recurrence (8), and all other cases, including ours, had a good prognosis without recurrence or distant metastasis.
The exact pathogenesis of this disease is unclear. Some investigators suggested that the neoplastic cells of MCA were transformed from the cells of DCIS by the processes of metaplasia (16), along with negative expression of ER and PR. However, the tumor cells of DCIS were strongly positive for ER in this case. Others suggested that, mucinous metaplasia of epithelial cells and large cystic transformation could lead to the formation of MCA in the intraductal papillary carcinoma (17).
The incidence of the breast MCA is fairly low; thus, it should be distinguished from metastatic MCA from the ovary, pancreas, or appendix. Firstly, the detailed medical history of the patient and careful clinical examination of the abdomen and pelvis are necessary. The patient in this study underwent a general CT examination, and no tumor at other sites appeared at the same time as the breast mass was observed. Secondly, immunohistochemical staining with CK7 and CK20 is helpful for diagnosis. The MCA of ovary and pancreas is positive for CK7 and CK20; the appendix MCA is positive for CK20 but negative for CK7. The breast MCA is positive for CK7 and negative for CD20, which is consistent with our findings. Thirdly, the breast MCA needs to be differentiated from the breast MC. MC is characterized by clusters of tumor cells floating in an extracellular mucinous lake, and the cells are mild atypia and lack intracellular mucin. MC is positive for ER, PR, and CgA, and it has a low Ki67 ratio, different from the high proliferation index of MCA.
Through discussion, we learned that the breast MCA and breast mixed MCA and IDC occurred mainly in postmenopausal women with a high Ki67 proliferation index and triple-negative expression pattern, but they had an excellent prognosis. Here, we present a case of mixed primary MCA and IDC of the breast and describe the results of histological examination, immunohistochemical staining and molecular detection in detail. Furthermore, we reviewed the literature and compare the clinical and pathological characteristics of the patient in this paper with others who had the breast MCAs. The differential diagnosis with other metastatic diseases is also introduced. Considering the molecular profile of triple-negative breast carcinoma, we used next generation sequencing to evaluate BRCA1 and BRCA2 mutation in the current case, but no mutation is found. Detailed genetic analysis of more cases could reveal the molecular basis of this rare tumor. The limitation of this article is that the aetiology of coexistent MCA and IDC is unknown.
Conclusions
MCA of the breast is a rare tumor, especially when it is mixed with IDC. All these reports about MCA are case reports. MCA has unique morphological and immunohistochemical characteristics. Accurate diagnosis of primary breast MCA requires detailed clinical and imaging information, in conjunction with complete surgical resection, histological evaluation, and immunohistochemical analysis to exclude metastasis. The true biological behavior of mixed MCA and IDC requires a long-term follow-up and evaluation of more patients.
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for the English language editing and review services.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1596/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1596/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koenig C, Tavassoli FA. Mucinous cystadenocarcinoma of the breast. Am J Surg Pathol 1998;22:698-703. [Crossref] [PubMed]
- Böcker W. WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics. Verh Dtsch Ges Pathol 2002;86:116-9. [PubMed]
- Allison KH, Brogi E, Ellis IO, et al. WHO classification of tumours editorial board. Breast tumours. 5th edition. Lyon: IARC, 2019.
- Deng Y, Xue D, Wang X, et al. Mucinous cystadenocarcinoma of the breast with a basal-like immunophenotype. Pathol Int 2012;62:429-32. [Crossref] [PubMed]
- Jain E, Kumar A, Jain R, et al. Primary Mucinous Cystadenocarcinoma of the Breast: A Rare Case Report With Review of Literature. Int J Surg Pathol 2021;29:740-6. [Crossref] [PubMed]
- Koufopoulos N, Goudeli C, Syrios J, et al. Mucinous cystadenocarcinoma of the breast: the challenge of diagnosing a rare entity. Rare Tumors 2017;9:7016. [Crossref] [PubMed]
- Kong J, Wang H, Zhang Q, et al. Case Report Primary mucinous cystadenocarcinoma of the breast coexisting with invasive ductal carcinoma: a case report and review of the literature. Int J Clin Exp Med 2017;10:7256-60.
- Nayak A, Bleiweiss IJ, Dumoff K, et al. Mucinous Cystadenocarcinoma of the Breast: Report of 2 Cases Including One With Long-Term Local Recurrence. Int J Surg Pathol 2018;26:749-57. [Crossref] [PubMed]
- Li X, Peng J, Zhang Z, et al. Mammary mucinous cystadenocarcinoma. Breast J 2012;18:282-3. [Crossref] [PubMed]
- Kim SE, Park JH, Hong S, et al. Primary Mucinous Cystadenocarcinoma of the Breast: Cytologic Finding and Expression of MUC5 Are Different from Mucinous Carcinoma. Korean J Pathol 2012;46:611-6. [Crossref] [PubMed]
- Lin LH, Hernandez O, Zhu K, et al. Genetic profile of primary mucinous cystadenocarcinoma of the breast-A case report. Breast J 2021;27:731-4. [Crossref] [PubMed]
- Rosen PP, Scott M. Cystic hypersecretory duct carcinoma of the breast. Am J Surg Pathol 1984;8:31-41. [Crossref] [PubMed]
- Witherspoon LE, Oxenhandler RW. A rare tumor: mucinous cystadenocarcinoma of the breast. Am Surg 2015;81:E106-8. [Crossref] [PubMed]
- Kamrani G, Nikbakhsh N, Hosseini A, et al. Mucinous cystadenocarcinoma of breast in a 69-year-old woman with positive hormone receptors, the first case reported. Caspian J Intern Med 2021;12:S444-6. [PubMed]
- Honma N, Sakamoto G, Ikenaga M, et al. Mucinous cystadenocarcinoma of the breast: a case report and review of the literature. Arch Pathol Lab Med 2003;127:1031-3. [Crossref] [PubMed]
- Chen WY, Chen CS, Chen HC, et al. Mucinous cystadenocarcinoma of the breast coexisting with infiltrating ductal carcinoma. Pathol Int 2004;54:781-6. [Crossref] [PubMed]
- Lee SH, Chaung CR. Mucinous metaplasia of breast carcinoma with macrocystic transformation resembling ovarian mucinous cystadenocarcinoma in a case of synchronous bilateral infiltrating ductal carcinoma. Pathol Int 2008;58:601-5. [Crossref] [PubMed]
- Sentani K, Tashiro T, Uraoka N, et al. Primary mammary mucinous cystadenocarcinoma: cytological and histological findings. Diagn Cytopathol 2012;40:624-8. [Crossref] [PubMed]
- Rakıcı S, Gönüllü G, Gürsel SB, et al. Mucinous Cystadenocarcinoma of the Breast with Estrogen Receptor Expression: A Case Report and Review of the Literature. Case Rep Oncol 2009;2:210-6. [Crossref] [PubMed]
- Petersson F, Pang B, Thamboo TP, et al. Mucinous cystadenocarcinoma of the breast with amplification of the HER2-gene confirmed by FISH: The first case reported. Hum Pathol 2010;41:910-3. [Crossref] [PubMed]
- Kucukzeybek BB, Yigit S, Sari AA, et al. Primary mucinous cystadenocarcinoma of the breast with amplification of the HER2 gene confirmed by FISH - case report and review of the literature. Pol J Pathol 2014;65:70-3. [Crossref] [PubMed]
- Seong M, Ko EY, Han BK, et al. Radiologic Findings of Primary Mucinous Cystadenocarcinoma of the Breast: A Report of Two Cases and a Literature Review. J Breast Cancer 2016;19:330-3. [Crossref] [PubMed]
- Domoto H, Terahata S, Yamazaki T, et al. Mucinous cystadenocarcinoma of the breast showing sulfomucin production. Histopathology 2000;36:567-9. [Crossref] [PubMed]
- Coyne JD, Irion L. Mammary mucinous cystadenocarcinoma. Histopathology 2006;49:659-60. [Crossref] [PubMed]
- Gulwani H, Bhalla S. Mucinous cystadenocarcinoma: a rare primary malignant tumor of the breast. Indian J Pathol Microbiol 2010;53:200-2. [Crossref] [PubMed]
- Lin DL, Hu JL, Shao SH, et al. Primary mucinous cystadenocarcinoma of the breast with endocervical-like mucinous epithelium. Breast Care (Basel) 2013;8:445-7. [Crossref] [PubMed]
- Valdespino VE, Matamoros IL, Valle ML, et al. Mucinous cystadenocarcinoma of breast, case report. Obstet Gnecol Rep 2019;3: [Crossref]
- Wang X, Li Y, Zhao P, et al. Primary mucinous cystadenocarcinoma of the breast: a clinicopathologic analysis of one case and review of the literature. Int J Clin Exp Pathol 2020;13:2562-8. [PubMed]
- Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat 2008;111:541-7. [Crossref] [PubMed]



